Oxidation-reduction reactions that occur in the presence of hydrogen peroxide (aqueous solution of hydrogen peroxide - H2O2(aq)) constitute a special case that must be analyzed separately, mainly regarding its balance. This is because the oxygens in hydrogen peroxide, which have Nox equal to -1, can either oxidize or reduce.
For example, let's look at two cases in which it behaves first as an oxidizing agent (reducing) and then as a reducing agent (oxidizing):
- oxidizing agent: whenever hydrogen peroxide is reduced, acting as an oxidizing agent, it generates water as a product.
If we add a hydrogen peroxide solution to a solution containing iodide ions (I-) in an acidic medium, we will have:
H2O2(aq) + I-(here) +H+(here) → H2O(1) + I2(s)
See that water and iodine are formed. But to check whether the hydrogen peroxide actually acted as an oxidizing agent and reduced, observe the determination of the oxidation numbers (NOx):*

The oxygen Nox of hydrogen peroxide decreased from -1 to -2, given that it received 1 electron. However, as we have two oxygens in each hydrogen peroxide molecule (H
2O2), the Nox variation will be equal to 2.So, as shown in the text “Redox balancing”, a necessary step to balance the reactions by the oxidation-reduction method is to invert the values of the variations of the Nox by the coefficients, being, in this case, as follows:
* H2O2 = 2 (∆Nox) = 2 → 2 will be the coefficient of the I-;
* I-= ∆Nox = 1 → 1 will be the coefficient of H2O2.
Thus, we have:
1 hour2O2(aq) + 2 I-(here) + H+(here) → H2O(1) + I2(s)
Hitting the other coefficients by balancing by trials:
- Since there are two oxygen atoms in the 1st member, the coefficient of water in the 2nd member must equal 2. And since there are also two iodide ions in the 1st member, the iodine coefficient in the 2nd member will be 1. Don't forget that we have to multiply the index by the coefficient to find the correct amount of atoms and ions in each member:
1 hour2O2(aq) + 2 I-(here) +H+(here) → 2 H2O(1) + 1 I2(s)
Do not stop now... There's more after the advertising ;)
- Now it only remains to balance the hydrogen cation of the 1st member, and its coefficient will have to be equal to 2, because in the 2nd member it has 4 hydrogens and in the 1st member it already has two:
1 hour2O2(aq) + 2 I-(here) +2 H+(here) → 2 H2O(1) + 1 I2(s)
- reducing agent: whenever hydrogen peroxide oxidizes, acting as a reducing agent, it generates oxygen (O2) as a product.
An example where hydrogen peroxide is reducing is when it comes into contact with potassium permanganate (KMnO4). This substance has a very characteristic violet color, but when it comes into contact with hydrogen peroxide it becomes colorless. This is because all manganese present in the MnO ion4- of the permanganate solution is reduced, giving rise to the Mn ion2+, as shown below:
+1 -1 +7 -2 +1 0 +2 +1 -2
H2O2 + MnO4-+ H+ → The2 + Mn2++ H2O
Calculating the Nox, we see that the oxygen in hydrogen peroxide actually oxidizes and causes the reduction of manganese:
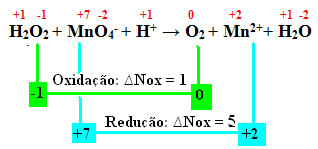
As in the previous example, the ∆Nox of hydrogen peroxide will be equal to 2, as there are two oxygens and each one loses an electron. Therefore, we have:
* O2 = 2 (∆Nox) = 2 → 2 will be the coefficient of MnO4-;
*MnO4- = ∆Nox = 5 → 5 will be the coefficient of the O2.
And like all O2 comes from hydrogen peroxide, the two substances have the same coefficient:
5 H2O2 + 2MnO4-+ H+ → 5 O2 + Mn2++ H2O
Balancing by the trial method, we have:
5 hours2O2 + 2 MnO4-+ 6 H+ → 5 O2 + 2 Mn2++ 8 H2O
* For any questions about how to calculate the oxidation number (Nox) of atoms and ions in a reaction, read the text “Determination of the Oxidation Number (Nox)”.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Oxidation-reduction reactions involving hydrogen peroxide"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-oxirreducao-envolvendo-agua-oxigenada.htm. Accessed on June 28, 2021.