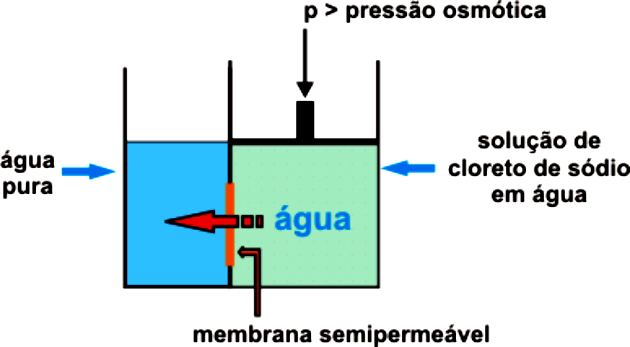
THE osmotic pressure it is a colligative property that corresponds to the pressure that must be exerted on a system to prevent osmosis from occurring spontaneously.
Osmosis is the passage of water from a less concentrated (hypotonic) to a more concentrated (hypertonic) medium through a semipermeable membrane until equilibrium is reached.
To prevent osmosis from starting and happening naturally, it is necessary to apply external pressure on the more concentrated solution, preventing the passage of the solvent to the more concentrated medium. That's the osmotic pressure.
The more concentrated the solution, the greater the osmotic pressure should be. Therefore, the osmotic pressure is proportional to the solute concentration.
How to calculate osmotic pressure?
Each solution has a different osmotic pressure value. Osmotic pressure can be calculated from the following formula:
π = M. A. T. i
Where, we have the following variables:
π = osmotic pressure;
M = concentration in mol/L;
R = universal gas constant, whose value corresponds to 0.082 atm. L. mol-1. K-1 or 62.3 mm Hg L. mol-1. K-1;
T = temperature on the absolute scale (Kelvin);
i = Van’t Hoff factor, which comprises the relationship between the total number of final and initial particles in ionic solutions.
Exercise solved
1. (Puccamp-SP) Occasionally, the 0.30 M glucose solution is used in an intravenous injection, as it has an osmotic pressure close to that of the blood. What is the osmotic pressure, in atmospheres, of the said solution at 37°C?
a) 1.00.
b) 1.50.
c) 1.76.
d) 7.63.
e) 9.83.
Considering the data provided by the question, we have:
M = 0.30 mol/L;
R = 0.082 atm. L. mol-1. K-1
T = 37° + 273 = 310 K
You must now apply these values to the formula for calculating the osmotic pressure:
π = M. A. T. i
π = 0,30. 0,082. 310
π = 7.63 atm (Alternative)
Classification of solutions
The solutions can be classified into three types, according to osmotic pressure:
- hypertonic solution: Has higher osmotic pressure and solute concentration.
- isotonic solution: When the solutions have the same osmotic pressure.
- hypotonic solution: Has lower osmotic pressure and solute concentration.

The importance of osmotic pressure for living beings
Saline is a substance prepared based on the principles of osmotic pressure. It should be applied at an osmotic pressure equal to that found in the body, this prevents the Red Cells do not suffer hemolysis or become shriveled.
The osmotic pressure of blood is approximately 7.8 atm. Therefore, for the correct functioning of the organism, the red blood cells must have the same osmotic pressure, guaranteeing the normal flow of water in and out of the cells.
In cases of dehydration, for example, the use of saline solution is indicated, which must be isotonic in relation to blood cells and other body fluids.
Saline has the function of returning the osmotic balance inside the body. That's because during dehydration, the blood becomes more concentrated than the inside of the cells, causing them to wither.
Osmosis and Reverse Osmosis
As we have seen, the osmosis it is the process of passing water from the hypotonic to the hypertonic environment, through a semi-permeable membrane, until the equilibrium between the concentrations is reached.
Meanwhile, the reverse osmosis it is a process of separating substances through a membrane that retains the solute. The solvent flows from the more concentrated medium to the less concentrated one and is isolated from the solute by a membrane that allows its passage.
This only happens due to the pressure exerted, making the semi-permeable membrane only allow the passage of water, retaining the solute. This pressure must be greater than the natural osmotic pressure.
For example, if the osmotic pressure applied is greater than necessary, reverse osmosis will occur. Thus, the flow will pass from the medium with the highest concentration to the one with the lowest concentration.