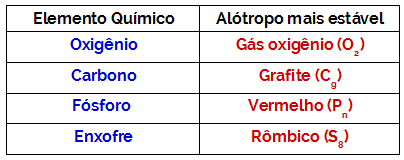
Solute and solvent are the two components of a homogeneous mixture called a chemical solution.
- Solute: is the substance that is dispersed in the solvent. It corresponds to the substance that will be dissolved and usually presents itself in a smaller amount in the solution.
- Solvent: is the substance in which the solute will be dissolved to form a new product. It presents itself in greater quantity in the solution.
Dissolution between the solute (dispersed) and the solvent (dispersant) occurs through interactions between their molecules.
The difference between these two components of a solution is that the solute is the substance that will dissolve and the solvent is the substance that will effect dissolution.
The best known solvent is water, considered the universal solvent. That's because it has the ability to dissolve a large amount of substances.
Solvent and Solvent Examples
See some examples of chemical solutions and discover the solutes and solvents of each one of them:
Water and salt
- Solute: Table salt - Sodium chloride (NaCl)
- Solvent: Water
As it is an ionic compound, the sodium chloride in the solution dissociates and forms ions which, in turn, are solvated by molecules of Water.
The positive pole of water (H+) interacts with the anion of salt (Cl-) and the negative pole of water (O2-) interacts with the cation (Na+).
This is a type of electrolytic solution, as the ionic species in solution are capable of conducting an electrical current.
water and sugar
- Solute: Sugar - Sucrose (C12H22O11)
- Solvent: Water

Sugar is a covalent compound and when dissolved in water the molecules they disperse but do not change their identity.
This aqueous solution is classified as non-electrolytic, as the solute dispersed in the solution is neutral and, therefore, does not react with water.
Vinegar
- Solute: Acetic acid (CH3COOH)
- Solvent: Water

Vinegar is a solution that contains at least 4% acetic acid, a carboxylic acid which, being polar, interacts with water, also polar, through hydrogen bonds.
An important rule for solubility is that like dissolves like. Polar compounds are dissolved in polar solvents, while non-polar substances dissolve in non-polar solvents.
Other solutions
In addition to liquid solutions, there are also gaseous and solid solutions.
The air we breathe is an example of a gaseous solution, whose gases in greater quantity are nitrogen (78%) and oxygen (21%).
At metal alloys they are solid solutions. For example, brass (zinc and copper) is a mixture used to make musical instruments.
Want to gain more knowledge? So read these other texts:
- Chemical Solutions
- intermolecular forces
- Homogeneous and heterogeneous mixtures
What is the Solubility Coefficient?
The solubility coefficient is the limit of solute added to the solvent at a given temperature to form a saturated solution.
O solubility coefficient varies according to conditions, and can increase or decrease with changes in temperature and the solute in question.
There is a limit to which the solvent can dissolve.
Example: If you put sugar in a glass of water, at the first moment, you will notice that the sugar disappears in the water.
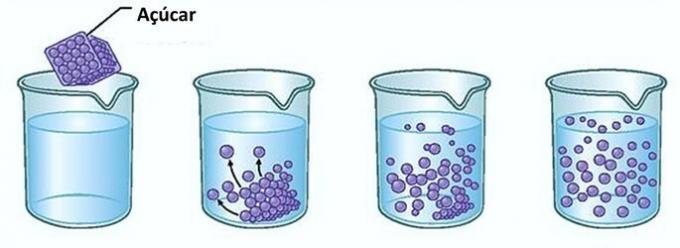
However, if you continue to add sugar, you will find that at some point it will start to accumulate in the bottom of the glass.
This is because the water, which is the solvent, has reached its limit of solubility and the maximum amount of concentration. The solute that remains at the bottom of the container and does not dissolve is called background body.
Excess sugar in the bottom of the glass will not dissolve and will not influence the concentration of the solution. Furthermore, the sugar deposited in the bottom of the glass will not make the water any sweeter.
Classification of solutions
Solutions can be classified by the amount of dissolved solute. Thus, they can be of three types: saturated, unsaturated and supersaturated.
- saturated solution: The solution has reached the limit of the solubility coefficient, that is, there is a maximum amount of solute dissolved in the solvent at a certain temperature.
- unsaturated solution: The amount of dissolved solute has not yet reached the solubility coefficient. This means that more solute can be added.
- supersaturated solution: There is more dissolved solute than under normal conditions. In this case, they present a precipitate.
To learn more about solutions, read the following texts.:
- Dilution of solutions
- Molality
- Molarity
- Titration
Concentration of solutions
From the solute and solvent it is possible to calculate the concentration of a solution.
Common concentration is defined as the ratio of the mass of solute dissolved in a given volume of solution.
Concentration calculation is done using the following formula:
Being,
Ç: Concentration (g/L);
m: mass of solute (g);
V: solution volume (L).
Example:
(Faap) Calculate the concentration, in g/L, of an aqueous solution of sodium nitrate containing 30 g of salt in 400 mL of solution:
Resolution:
Observe information regarding solute and solvent amounts. There are 30 g of salt (solute) in 400 mL of aqueous solution (solvent).
However, the volume is in mL and we need to transform it to L:
Now, to know the concentration, you just have to apply the formula:
With this result, we came to the conclusion that when we mix 30 g of salt with 400 mL of water, we will obtain a solution with a concentration of 75 g/L.
For more information on how to calculate the common concentration, these texts will be helpful.:
- Solution Concentration
- Exercises on Common Concentration