Thermal equilibrium, also called thermodynamic equilibrium, is when two bodies or substances reach the same temperature.
This concept of thermodynamics is related to the spontaneous heat transfer (thermal energy) that occurs between two bodies in contact.
In this process, the warmer body transfers heat to the cooler body until both are at the same temperature.

heat transfer scheme
The exchange of energy between two bodies (heat energy) results in the loss of thermal energy from the warmer body and the gain in energy from the cooler body.
Example
As an example, we can mention the mixture of hot coffee with cold milk. Although they have different initial temperatures, in a short time the hottest body (coffee) transfers thermal energy to the coldest one (milk). Thus, the mixture becomes warm, as a result of thermal equilibrium.
Assuming that coffee was at 50°C and milk at 20°C, thermal equilibrium is reached when both are at 35°C.

heat spread
It is important to note that heat is the exchange of energy and its transfer can occur in three ways:
- thermal conduction: increase in kinetic energy;
- thermal convection: creation of convection currents;
- thermal irradiation: by means of electromagnetic waves.
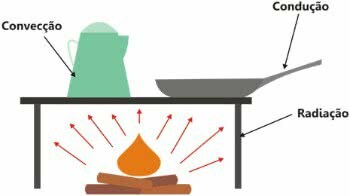
Types of Heat Propagation
Read more: heat spread.
Formula
To calculate the thermal balance, the following formula is used:
Q1 + Q2 + Q3... = 0 or ΣQ=0
Being,
Q: amount of heat (body temperature)
Thus, we know that the sum of all thermal energies is nil.
O sensible heat it is related to the temperature variation of bodies. It is calculated by the following formula:
Q = m. ç. Δθ
Where,
Q: amount of sensible heat (lime or J)
m: body mass (g or kg)
ç: specific heat of the substance (cal/g°C or J/Kg°C)
Δθ: temperature variation (°C or K)
Also read aboutCalorimetryandHeat and Temperature.
Entrance Exam Exercises with Feedback
1. (Mackenzie) When we mix 1.0 kg of water (sensitive specific heat = 1.0 cal/g°C) at 70°C with 2.0 kg of water at 10°C, we get 3.0 kg of water at:
a) 10°C
b) 20°C
c) 30°C
d) 40°C
e) 50°C
Alternative c) 30°C
2. (UFP-RS) Consider the following statements:
I. When two bodies are in thermal equilibrium, they both have the same amount of heat.
II. When two bodies are in thermal equilibrium, they both have the same temperature.
III. Heat is the transfer of temperature from one body to another.
IV. Heat is a form of energy in transit.
From the above statements, it can be said that:
a) I, II, III and IV are correct
b) I, II, III are correct
c) I, II and IV are correct
d) II and IV are correct
e) II and III are correct
Alternative d) II and IV are correct
3. (FATEC-SP) A system A is in thermal equilibrium with another B and this one is not in thermal equilibrium with another C. So, we can say that:
a) systems A and C have the same amount of heat.
b) the temperature of A is different from that of B.
c) systems A and B have the same temperature.
d) the temperature of B is different from that of C, but C can have the same temperature as in system A.
e) none of the above.
Alternative c) systems A and B have the same temperature.
4. (UFV-MG) When two bodies of different materials are in thermal equilibrium, isolated from the environment, it can be said that:
a) the hottest is the one with the least mass.
b) despite the contact, their temperatures do not vary.
c) the hottest provides heat to the coldest.
d) the coldest provides heat to the hottest
e) their temperatures depend on their densities.
Alternative b) despite the contact, their temperatures do not vary.
5. (UFScar-SP) Two bodies A and B, with masses mTHE inB, are initially at temperatures tTHE and youB, respectively, with tTHE tB. At a given moment, they are brought into thermal contact. After reaching thermal equilibrium, we will have:
a) t’a > t’b
b) t’a c) t’a = t’b
d) n.d.a.
Alternative c) t’a = t’b
See also the definition ofBalance in Physics.