THE simple distillation is nothing more than a method of separation of mixtures, used specifically when we have a homogeneous mixture formed by a solid dissolved in a liquid, such as sodium chloride (NaCl) in water.

Sodium chloride can be obtained from simple distillation
It is necessary to understand that, in the simple distillation, two fundamental physical state transformations always occur, the vaporization (passage from liquid to gas) and the condensation (passage from steam to liquid).
In the laboratory, to perform a simple distillation, the following equipment is required:
Bunsen burner or heating plate (1): equipment that is used to heat the mixture;
Distillation flask (2): container where the mixture is packed to receive heating;
Universal support (3): equipment used to attach the claw;
Claw (4): equipment used to hold the distillation flask;
Thermometer (5): equipment used to measure the temperature during the experiment;
Stopper (*): wooden material to close the upper end of the distillation flask;
Water outlet (6): path through which the heated water exits;
Water inlet (7): path through which cold water enters;
Condenser (8): equipment where condensation occurs during the experiment;
Erlenmeyer or beaker (9): container where the distilled liquid will be collected.
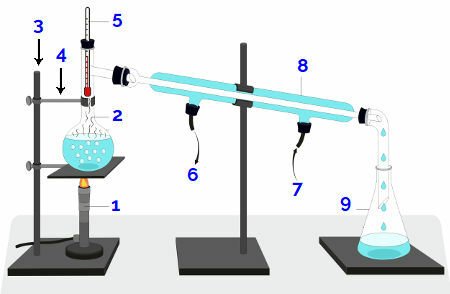
Schematic representation of a distiller
During the performance of a simple distillation, we have the following events:
First: the distillation equipment is organized;
Second: the mixture (eg water and sodium chloride) is added to the distillation flask;
Third: the heating of the mixture is started in the distillation flask using the Bunsen burner or the heating plate;
Room: the water starts to vaporize inside the distillation flask;
Fifth: the water vapor necessarily enters the distiller, since the upper part of the balloon is closed, turning into liquid again;
Sixth: The condensed liquid is then collected in the beaker or Erlenmeyer flask.

Representation of distilled liquid
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-destilacao-simples.htm
