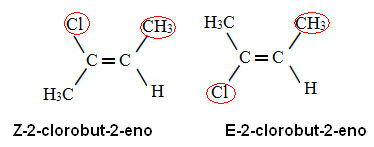
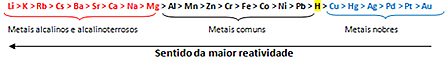
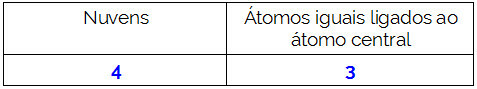
Is there a difference between gas and steam? If we only consider the visual aspects we will hardly notice such a differentiation. But in relation to chemical characteristics, it can be said that steam and gas are distinct due to possible changes in physical state.
Vapor is a reference to matter in the gaseous state. We say that this form is capable of being in equilibrium with the liquid or solid from which it is made by increasing the temperature.
Example: when you put water to boil, we get H2The in the steam state, corresponds to that little smoke that comes out of the kettle spout. If we want to turn this vapor into a liquid again, we will have condensation.
Do not stop now... There's more after the advertising ;)
Gas, in turn, is one of the physical states of matter. It has no defined shape or volume, it consists of an agglomerate of particles whose movements are random.
To liquefy a gas (turn it into a liquid) it is necessary to change its pressure.
Example: the gas in the cylinder (LPG gas = liquefied petroleum gas) is in a liquid state because of the enormous pressure inside the container in which it is contained.
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Difference between gas and steam"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/diferenca-entre-gas-vapor.htm. Accessed on June 27, 2021.