The oxidation-reduction reactions studied mainly in Physical Chemistry are those in which electron transfer occurs. The reacting species (atom, ion or molecule) that loses one or more electrons is the one that undergoes oxidation. The chemical species that receives electrons, on the other hand, is reduced.
Generally, when this type of reaction is studied in Inorganic Chemistry, it is called simple exchange reaction or of displacement.
For any reaction to take place it is necessary to satisfy certain conditions. One of them is that there must be chemical affinity between the reagents, that is, they must interact in such a way as to enable the formation of new substances.
In the case of redox reactions, affinity means that one of the reactants tends to gain electrons and the other tends to lose electrons. This trend corresponds to reactivity of the chemical elements involved.
Let's see how it is possible to compare the reactivity between metals.
Assume that we want to store a solution of copper II sulfate (CuSO
4). We could not possibly place this solution in an aluminum container, because the following reaction would occur:2 Al(s) + 3 CuSO4(aq)→ 3 Cu(s) + Al2(ONLY4)3(aq)
Note that aluminum has oxidized, losing 3 electrons each and becoming aluminum cation:
Al(s) → Al3+(here) + 3 and-
Simultaneously, the cation copper (Cu2+) that was present in the solution received electrons from aluminum and reduced, becoming metallic copper. Each copper cation receives two electrons:
Ass2+(here) + 2 and- → Cu(s)
However, if it were the other way around and we wanted to store a solution of aluminum sulphate (Al2(ONLY4)3(aq)), it wouldn't be a problem to put it in a copper container, as this reaction would not occur:
Ass(s) + Al2(ONLY4)3(aq) → does not occur
These observed facts can be explained by the fact that aluminum being more reactive than copper.
Metals have a tendency to give up electrons, that is, to oxidize. When comparing various metals, the one with the greatest tendency to donate electrons is the most reactive. Consequently, the reactivity of metals is also associated with their ionization energy, that is, the minimum energy needed to remove an electron from the gaseous atom in its ground state.
Do not stop now... There's more after the advertising ;)

Based on this, the metal reactivity queue or row of electrolytic voltages, shown below:
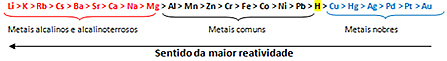
The most reactive metal reacts with ionic substances whose cations are less reactive. In other words, the metal on the left reacts with the substance formed by ions on its right. The opposite does not happen.
Remembering the example given, see in the reactivity row that aluminum (Al) is to the left of copper (Cu). Therefore, aluminum reacts with the solution formed by copper cations; but copper does not react with a solution formed by aluminum cations.
Note that the most reactive metal is lithium (Li) and the least reactive is gold (Au).

This is one of the reasons why gold is so valuable, because if it doesn't react, it stays intact for a long time. This can be seen in the gold-coated Egyptian sarcophagi and sculptures that date back to the most remote antiquity. We also see this when we compare the durability of a pure gold jewelry to jewelry made from other metals that are more reactive than gold.

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Order of Reactivity of Metals"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/ordem-reatividade-dos-metais.htm. Accessed on June 28, 2021.


