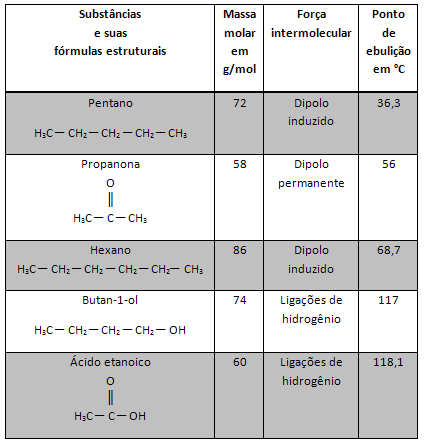
In the text "geometric isomer or cis-trans” was shown how geometric spatial isomerism or cis-trans. Briefly, the isomer cis corresponds to compounds whose equal ligands on each carbon atom are in the same plane; already in the isomer trans, they are on opposite sides.
This is identified by the origin of these terms, which comes from Latin, where cis means "next to" and trans "across".
This nomenclature is very useful when two carbons each have only two different ligands. However, these terms can be ambiguous when referring to alkenes, whose double bond carbon atoms have more than two different ligands in the set.
For example, consider the compound below:

Note that on carbon x the lowest atomic number ligand is CH3, and on carbon y is the H. But if we were to say that this compound is the isomer cis, the following question could arise: what's he like cis if the linking groups are equal (CH3) are on opposite sides?
Do not stop now... There's more after the advertising ;)
Therefore, to avoid this ambiguity, it is more correct to use in these cases the nomenclature E-Z, where the letter E comes from the German word
entgegen, which means “opposites” and Z comes from the German word zusammen, which means “together”. This nomenclature follows the following rule:
Applying this priority rule to 2-chlorobu-2-ene, we have that in carbon x the ligand with the highest atomic number is Cl, and on carbon y is the CH3. Thus, we have the following isomers:
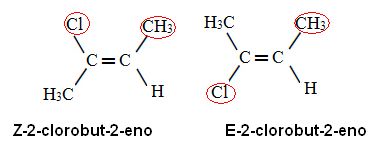
In the first case, the ligands with the highest atomic numbers are on the same side of the plane (Z) and in the second, they are on opposite sides (E).
This also occurs with cyclic compounds. It is important to remember that it is not correct to relate the terms cis and Z, and trans and E, as they are different naming systems.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Isomery E-Z in place of cis-trans"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/isomeria-e-z-no-lugar-cis-trans.htm. Accessed on June 27, 2021.