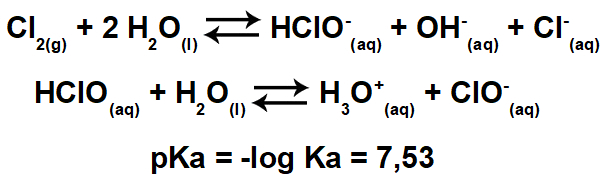Entropy is the measure of the degree of disorder of a system, being a measure of the unavailability of energy.
It is a physical quantity that is related to Second Law of Thermodynamics and that it tends to increase naturally in the Universe.
Definition of Entropy
“Disorder” should not be understood as “mess” but rather as a form of system organization.
The concept of entropy is sometimes applied in other areas of knowledge with this sense of disorder, which is closer to common sense.
For example, let's imagine three jars, one with small blue marbles, another with the same type of marbles but red, and the third empty.
We take the empty pot and put all the blue balls underneath and all the red balls on top. In this case, the balls are separated and arranged by color.
When shaking the pot, the balls began to mix in such a way that at a given moment there is no longer the initial separation.
Even if we continue to shake the pot, it is unlikely that the balls will return to the same initial organization. That is, the ordered system (balls separated by color) has become a disordered system (mixed balls).

Thus, the natural tendency is to increase the disorder of a system, which means an increase in entropy. We can then say that in systems: ΔS >0, where S is entropy.
Also understand what it is enthalpy.
Entropy and Thermodynamics
The concept of Entropy began to be developed by the French engineer and researcher Nicolas Sadi Carnot.
In his research on the transformation of mechanical energy into thermal, and vice versa, he found that it would be impossible for there to be a fully efficient thermal machine.
THE First Law of Thermodynamics basically determines that "energy is conserved". This means that in physical processes energy is not lost, it is converted from one type to another.
For example, a machine uses energy to do work and in that process the machine heats up. That is, mechanical energy is being degraded into thermal energy.
Thermal energy does not change back into mechanical energy (if that happened the machine would never crash), so the process is irreversible.
Later, Lord Kelvin complemented Carnot's research on the irreversibility of thermodynamic processes, giving rise to the foundations of Second Law of Thermodynamics.
Rudolf Clausius was the first to use the term Entropy in 1865. Entropy would be the measure of the amount of Thermal energy that cannot be reversed into mechanical energy (cannot do work) at a certain temperature.
Clausius developed the mathematical formula for the variation of entropy ()S) that is currently used.
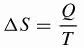
Being,
ΔS: entropy variation (J/K)
Q: heat transferred (J)
T: temperature (K)
Read too:
- Thermodynamics
- Carnot Cycle
- Energy
- Types of Energy
- Physics Formulas
Solved Exercises
1) Enem - 2016
Until 1824 it was believed that thermal engines, examples of which are steam engines and current combustion engines, could have an ideal operation. Sadi Carnot demonstrated the impossibility of a thermal machine, operating in cycles between two thermal sources (one hot and one cold), to obtain 100% efficiency. Such limitation occurs because these machines
a) perform mechanical work.
b) produce increased entropy.
c) use adiabatic transformations.
d) contravene the energy conservation law.
e) operate at the same temperature as the hot source.
Alternative: b) produce increased entropy.
2) Enem - 2011
A motor can only do work if it receives an amount of energy from another system. In this case, the energy stored in the fuel is, in part, released during combustion so that the appliance can function. When the engine runs, some of the energy converted or transformed in combustion cannot be used to do work. This means that there is energy leakage in another form. Carvalho, A. X. Z.
Thermal Physics. Belo Horizonte: Pax, 2009 (adapted).
According to the text, the energy transformations that occur during engine operation are due to a
a) heat release inside the engine is impossible.
b) work performed by the engine is uncontrollable.
c) full conversion of heat to work is impossible.
d) transformation of thermal energy into kinetics is impossible.
e) potential energy use of the fuel is uncontrollable.
Alternative: c) full conversion of heat to work is impossible.
See too: Exercises on Thermodynamics