Osmosis is a Colligative Property conceptualized as the passage of solvent through semi-permeable membranes. Hence the meaning of the Greek origin of its name: osmos = impulse. In this process, there is a diffusion of solvent from the less concentrated (or more diluted) solution to the more concentrated (less diluted), thus equaling the concentration of both solutions.
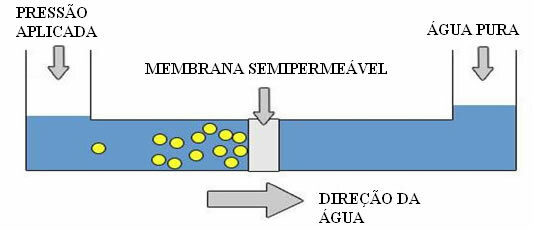
However, mainly in seaside regions, which have little drinking water, a technique is used to transform salt water into fresh water, that is, in the opposite direction to the described osmosis. Therefore, it is called reverse osmosis or reverse osmosis (or reverse osmosis). In this process, the solvent passes through the semipermeable membrane from the most concentrated solution to the least concentrated solution.
With this objective of desalinating sea water, many plants have been built, such as the one in Yuma in Arizona (United States), which has the capacity to produce 72 million gallons of pure water per day. In 2010 the largest desalination plant in the world was inaugurated in Israel. Built to produce 127 million cubic meters of water a year – enough to supply one-sixth of the Israeli population.
In Brazil, examples are the Greek islands, the islands of Fernando de Noronha, Easter Island and the island of Malta. In addition to using this process in brackish water (which comes from the subsoil, containing a lot of salt) in certain regions of northeastern Brazil.
But how is this possible? This is due to the osmotic pressure, that is, the external pressure that is applied to the solution, to prevent the entry of pure water. If this pressure is greatly increased, reverse osmosis is obtained, in which there is a passage of water from the solution to pure water.
Do not stop now... There's more after the advertising ;)
Reverse Osmosis Scheme
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Physicochemical - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Reverse Osmosis in the desalination of sea waters"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/osmose-reversa-na-dessalinizacao-das-aguas-dos-mares.htm. Accessed on June 28, 2021.