Reversible reactions usually start with a certain amount of reagents. As the direct reaction starts, over time, these reagents are consumed for the formation of the products, consequently the concentration of the reagents decreases while the concentration of the products increases. Then, the inverse reaction also starts, producing the reactants as well, until the rate of development (speed) of the direct and inverse reactions remains the same, reaching the so-called chemical balance.
In equilibrium, there is the equilibrium constant Kc, which is basically expressed by:
|
Kc = [products]coefficient in balanced chemical equation [reagents]coefficient in balanced chemical equation |
That is, considering the following generic equilibrium reaction:
a A + b B ↔ c C + d D
Since the lowercase letters are the coefficients, and the uppercase letters are the substances, the equilibrium constant of this reaction will be:
Kc = [Ç]ç. [D]d
[THE]The. [B]B
More details about this can be seen in the text Kc and Kp equilibrium constants. This text also shows us something important: that Kc values can show us whether the concentration of the reagents and the products are equal or if one is greater than the other and, as a consequence, if the chemical balance is shifted in some direction of the reaction.
So we need to determine the value of Kc. To do this, keep in mind that these calculations are experimental, so let's look at some examples of reactions and the data obtained from them.
Something that is very helpful in performing these calculations is to write a table similar to the one shown below and follow the steps mentioned in it:

Table to organize the data used to calculate the equilibrium constant
Now, let's go to practice:
Example 1: In a closed container, with a capacity of 2 L, at a temperature of 100°C, there is 20 mol of N2O4. The following reversible reaction begins to occur: N2O4 ↔ NO2. After some time, it was found that the reaction reached chemical equilibrium and that 8 mol of NO2 had formed. What is the value of the equilibrium constant Kc at a temperature of 100°C?
Do not stop now... There's more after the advertising ;)
Resolution:
Let's use the table:

Table used to solve equilibrium constant calculation example
Note that in the line where the quantities that react and form were written, we know that 4 moles of N were spent2O4, because the ratio is 1: 2, and that 8 mol of NO were formed2.
Now just replace the values found in the expression of the equilibrium constant Kc of this reaction:
Kc = [AT THE2]2
[N2O4]
Kc = (4 mol/L) 2
(8 mol/L)
Kc = 2 mol/L
The value of Kc is dimensionless, it has no unit related to any magnitude.
Now, let's look at an example, which also contains products from the beginning:
Example 2: In a closed container, with a capacity of 5 L, at temperature T, there are 2 moles of hydrogen gas, 3 moles of iodine gas and 4 moles of hydrogen iodide. The reaction enters chemical equilibrium, at temperature T, and it turns out that there is 1 mole of hydrogen gas in the vessel. What is the graph representing this equilibrium and what is the value of the equilibrium constant Kc at the temperature of T?
Resolution:
Using table:
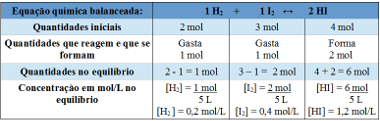
Table used to determine the equilibrium constant
The graph showing the variations in mol/L concentrations of reagents and products until they reach equilibrium can be given by:

Chemical equilibrium graph showing changes in concentrations of reagents and products
Now we discover the value of the equilibrium constant:
Kc =__[HI]2__
[H2 ]. [I2]
Kc = (1,2)2
0,2. 0,4
Kc = 18
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Calculation of the equilibrium constant Kc"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/calculo-constante-equilibrio-kc.htm. Accessed on June 28, 2021.
Chemistry

Test your knowledge and learn more with this list of solved exercises on chemical balances. Through this material, you will be able to better understand how to work equilibrium constants (Kp, Kc and Ki), equilibrium shift, pH and pOH, as well as equilibrium in so-called buffer solutions.