At organic addition reactions are very common and studied. It is worth remembering that this is a type of reaction that presents as a basic and predominant mechanism the break the link, or links, pi so that two or more atoms can start to be part of the chain.
An example of using addition reactions is related to margarine production. This product, so common in people's daily lives, is formed from the hydrogenation (an addition reaction) of vegetable oils, which present pi links in its constitution.
The types of addition reactions are:
hydrogenation (addition of hydrogen atoms);
Halogenation (addition of halogen atoms: Cl2, br2, I2 and F2);
Reaction with halide (addition of halogen-containing inorganic hydracids such as HCl, HI, Hbr and HF);
Hydration (addition of a hydronium, H+, and a hydroxyl, OH-).
During an addition by hydration or with halide, The Markovnikov's rule it is crucial for us to predict the products that will be formed. In this rule, we have that the atom of hydronium (H+) from inorganic hydroxide or water will be added to the
more hydrogenated carbon of the double bond. already the halogen (Cl, Br, I, F) of the halide or the hydroxyl (OH-) of water will be added to the less hydrogenated carbon of the pi link. See an example of applying this rule in the following equation: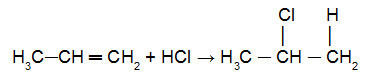
Reaction of proprene with HCl
In this equation, we can see that the atom of (Cl) was added to the less hydrogenated carbon of the pi link, while the atom of hydronium (H+) was added to the most hydrogenated carbon atom of the pi link.
there is only one exception to Markovnikov's rule: the Kharash reaction. In it an inversion occurs, that is, the atom of hydronium (H+) will be added to less hydrogenated carbon gives pi link, it's the halogen (Br) of the halide will be added to the more hydrogenated carbon gives pi link. The detail is that this reaction only occurs in one way:
Presence of organic peroxide;
Use of Hbr.
NOTE: if these conditions are not met, the Markovnikov's rule will be used normally.
See below an application of the Kharash reaction in propylene:
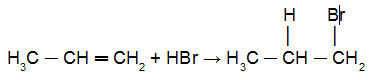
Reaction of propene with HBr in the presence of peroxide
NOTE: It is worth noting that an organic peroxide is a compound that necessarily has the group (R─O─O─R) in its constitution. The most used organic peroxide in Kharash reactions is derived from carboxylic acids and has the following structure:
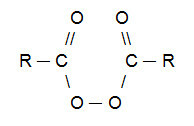
General structural formula of an organic peroxide
The explanation for the Kharash reaction to invert the Markovnikov rule lies in the fact that initially the peroxide is broken down (step 1), forming free radicals with two oxygen atoms that attack the hydrogen of HBr (step 2). For this reason, who will attack the alkene molecule initially will be Br (step 3). Only after the hydrogen will bind to the alkene chain. Follow the sequence of facts:
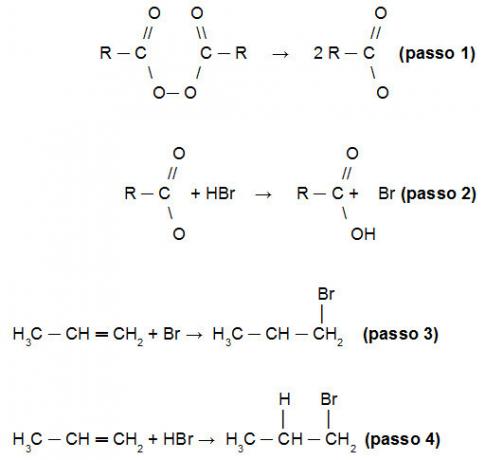
Demonstration of the mechanism of the Kharash reaction
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacao-kharash.htm
