Gas transformations are processes in which a gas may have one or more of its parameters from pressure, volume and temperaturechanged. There are special gaseous transformations in which at least one of these quantities is kept constant.
The types of gas transformations are:
isobaric transformation;
isothermal transformation;
isovolumetric transformation;
adiabatic transformation.
See too:Thermology — the study of phenomena linked to heat and temperature
isothermal transformation
The isothermal transformation is one in which the temperature of a gas remains constant. In this type of transformation, pressure and volume areinverselyproportional, so that by increasing the pressure, the volume is reduced and vice versa.
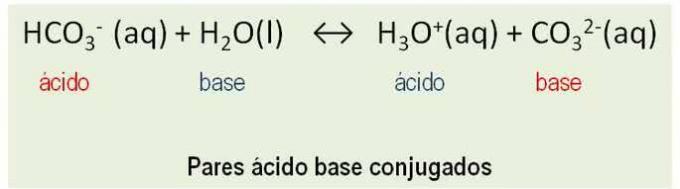
Isothermal transformations are described by Lhey boyle-mariotte. According to this law, the product between pressure and volume of an ideal gas is constant:

FORi and FORf – initial and final pressure
Vi and Vf – initial and final speed
K – constant
isobaric transformation
The isobaric transformation is characterized by a
volume variation and temperature, keeping the pressure constant. This type of transformation is mathematically described by the law of Charles and Gay-Lussac. During an isobaric transformation, temperature and volume are directly proportional, that is, keeping constant the pressure of a gas, the volume occupied by it will increase with the increase of the temperature of the gas. gas.See the formula used by Gay-Lussac law:
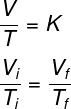
Ti and Tf – initial and final temperature
According to the Gay-Lussac's Law, the ratio between the volume and pressure of a gas, during an isobaric transformation, is equal to a constant.
Do not stop now... There's more after the advertising ;)
Isovolumetric transformation
Isovolumetric, isometric or isochoric transformation is the name of the process in which an ideal gas undergoes changes in pressure and temperature, maintaining itself constant your volume. This type of gas transformation, which takes place inside rigid walled containers, is mathematically defined by the Charles' law.
According to this law, the ratio between pressure and temperature of an ideal gas is always constant. Furthermore, in this type of transformation, pressure and temperature are directly proportional: doubling the pressure, we double the temperature and vice versa.
The formula that describes the behavior of ideal gases during isovolumetric transformations is as follows:
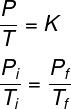
Read too: Thermodynamics — an area of Physics that studies phenomena in which there is heat exchange
adiabatic transformation
Adiabatic transformation is the name of the process undergone by a gas that doesn't change heat with the external environment or with the walls of its container. The formula that mathematically explains the behavior of an ideal gas that undergoes an adiabatic transformation states that the product between pressure and volume raised to a constant γ is constant.

The constant γ depends on the degreesinfreedom of the gas, that is, the number of directions in which the gas molecules can move. It can also be calculated by the ratio between the specific heat in pressureconstant and heatspecific in volumeconstant.
Examples of gas transformations
isothermal transformation – any slow expansion or contraction of a gas that occurs at a constant temperature.
isobaric transformation – cooling the gas contained in a bladder placed to cool in a refrigerator.
Isovolumetric transformation – heating the water vapor confined in a pressure cooker completely.
adiabatic transformation – gas being expelled by an aerosol spray.

See too: Calorimetry – formulas, concepts and solved exercises
Solved exercises on gas transformations
Question 1 — Review the alternatives below and check the answer that contains only true alternatives.
I – Isothermal transformations are those in which the gas temperature is kept constant.
II – Adiabatic transformations involve heat exchange between the gas and the external environment.
III – Isochoric transformation is one in which the gas pressure is kept constant.
IV – Isobaric transformations happen with constant pressure.
Are correct:
a) I and II.
b) I, II and III.
c) II and III.
d) I and IV.
Resolution:
Alternatives II and III are wrong, since adiabatic transformations do not involve heat exchange, and isochoric transformations are those that take place in constant volumes. Thus, the correct alternative is the letter D.
Question 2 —An ideal gas undergoes a rapid transformation, so that its temperature, pressure and volume undergo sudden changes in a short period of time. According to his knowledge of gas transformations, the transformation undergone by the gas was:
a) isothermal.
b) isobaric.
c) adiabatic.
d) isovolumetric.
Resolution:
Adiabatic transformations occur quickly, so there is no time for the gas to exchange heat with the external environment. In this type of transformation, the pressure, volume and temperature parameters change abruptly. Thus, the correct alternative is the letter C.
By Rafael Hellerbrock
Physics teacher
Would you like to reference this text in a school or academic work? Look:
HELERBROCK, Rafael. "Gas transformations"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/transformacoes-gasosas.htm. Accessed on July 27, 2021.