Experiments are a practical way to learn and test your knowledge of the concepts studied in Chemistry.
Take advantage of these chemical experiments, which can be done at home (under adult supervision) or worked on in the classroom with the teacher, to complement your studies.
1st experience - unraveling the colors
Concepts involved: chromatography and separation of mixtures
Materials
- pens (marked markers) of various colors
- alcohol
- coffee filter paper
- cup (glass to facilitate the monitoring of the experiment)
How to make
- Use scissors and cut strips of filter paper. For each pen used, make a rectangle.
- Now, at a distance of approximately 2 cm from the base, draw a circle with your chosen color pen and paint all the inside.
- Glue the edge of the paper farthest from the drawn marble onto a support. For this, you can use a tape and attach it to a pencil.
- Add alcohol to the cup, not too much, as it should just touch the end of the paper near the pen mark.
- Place the paper in the cup so that it is vertical. The pencil that supports it should be resting on the edges.
- Wait between 10 and 15 minutes for the alcohol to rise through the filter paper. After that, remove the papers and let them dry.
Result
When alcohol passes through the pen mark, it interacts with the color components and drives them across the paper. Thus, the different pigments will be separated by contact with alcohol.
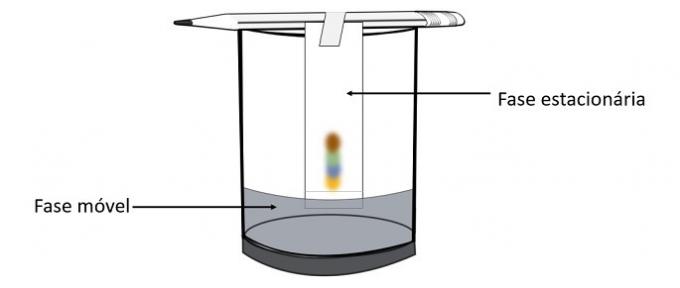
Through this experiment it is possible to know which colors were mixed to create the color of the pen.
Explanation
Chromatography is a type of process for separating mixtures. Filter paper is the stationary phase and alcohol is the mobile phase that drags the components of the mixture as it passes through the stationary phase. In this process, the greater the interaction with the alcohol, the faster the pigment will move with the passage of the solvent.
The constituents of the material, as they have different properties, will interact with the mobile phase in different ways, which can be noticed by the different drag times in the stationary phase.
Learn more about chromatography.
2nd experience - food preservation
Concepts involved: organic compounds and chemical reactions
Materials
- apple, banana or pear
- lemon or orange juice
- Vitamin C tablet
How to make
- Choose one of the three fruits and cut it into 3 equal parts.
- The first piece will serve as a comparison with the others. So don't add anything to it, just leave it exposed to air.
- Into one of the pieces, drip the contents of a lemon or an orange. Spread so that the entire inside of the fruit is covered with juice.
- In the last part, spread the vitamin C, it can be a crushed tablet, all over the fruit pulp.
- Watch what happens and compare the results.
Result
The pulp of the fruit that has been exposed to the air should quickly darken. Lemon or orange juice and vitamin C, a chemical called ascorbic acid, should slow down the browning of the fruit.

Explanation
When we cut a fruit, its cells are damaged releasing enzymes such as polyphenol oxidase, which in contact with the air oxidize the phenolic compounds present in the food and cause an enzymatic browning.
To prevent the action of oxygen, preservatives such as ascorbic acid are used because they are preferentially oxidized in place of phenolic compounds. In addition to the vitamin C tablet, ascorbic acid is also present in natural sources, such as citrus, lemon and orange, suggested in the experiment.
Learn more about oxidation.
3rd experience – who freezes faster?
Concepts involved: colligative properties and cryoscopy
Materials
- two tubes (used for party favors)
- glass bowl
- table salt
- filtered water
- ice
- thermometer
How to make
- Add the same amount of filtered water to both tubes. For example, 5 mL in each tube.
- Add the table salt to one of the tubes and place an identification tape to distinguish which one is salty.
- Fill the glass container with crushed ice and add some salt.
- Place the two tubes simultaneously inside the ice and watch what happens.
- Record the freezing temperature for each situation.
Result
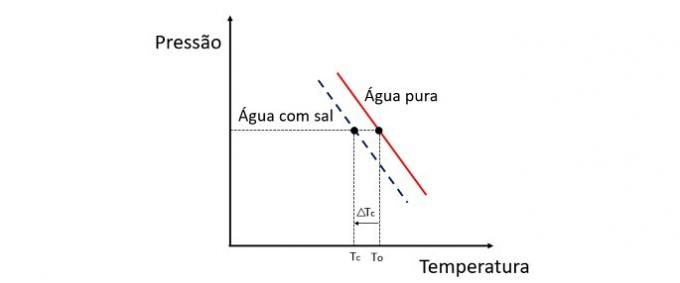
Adding a solute to water lowers the freezing temperature. Therefore, plain water tends to freeze much faster than a salt and water solution when exposed to the same conditions.
Explanation
Cryoscopy is a colligative property that studies the variation in the temperature of a solvent when different amounts of solute are dissolved in it.
Lowering the freezing temperature of water is caused by a non-volatile solute and this phenomenon has many practical applications. Therefore, the greater the concentration of solute in the solution influences the cryoscopic effect.
If, for example, water freezes at 0 °C and we add salt to it, the phase change temperature will be negative, ie much lower.
This is why sea water does not freeze in places whose temperature is below 0°C. Salt dissolved in water tends to further lower the freezing temperature. In places where there is snow, it is also common to throw salt on the roads to melt the ice and avoid accidents.
Learn more about colligative properties.
4th experiment - decomposition of hydrogen peroxide
concepts involved: chemical reaction and catalyst
Materials
- Half a raw potato and half cooked
- A piece of raw liver and another cooked piece
- Hydrogen peroxide
- 2 dishes
How to make
- In each dish add food, potatoes together and livers together.
- In each of the four materials add 3 drops of hydrogen peroxide.
- Watch what happens and compare the results.
Result
Hydrogen peroxide, a hydrogen peroxide solution, when it comes into contact with raw foods, begins to effervescence almost instantly.
This experiment can also be done by adding a piece of food to a container with hydrogen peroxide to make the reaction more noticeable.
Explanation
The effervescence presented by hydrogen peroxide when coming into contact with raw foods characterizes the occurrence of a chemical reaction, which is the decomposition of hydrogen peroxide and release of the gas oxygen.
The decomposition of hydrogen peroxide occurs through the action of the catalase enzyme, found in the peroxisomes organelle, present in animal and plant cells.
It is important to highlight that the decomposition of hydrogen peroxide occurs spontaneously, in the presence of sunlight, but in a very slow reaction. However, catalase acts as a catalyst, increasing the speed of the chemical reaction.
Hydrogen peroxide can be a toxic substance to cells. Therefore, catalase breaks down the compound and produces water and oxygen, two substances that do not harm the body.
When food is cooked, its components undergo changes. The modifications caused by cooking also compromise the action of catalase by denaturing the protein.
The same action we see with food is what happens when we put hydrogen peroxide on a wound. Catalase acts and there is the formation of bubbles, which consists in the release of oxygen.
Learn more about chemical reactions.
Bibliographic references
SAINTS, W. L. P.; MOL, G. S. (Coords.). Citizen Chemistry. 1. ed. São Paulo: New Generation, 2011. v. 1, 2, 3.
BRAZILIAN CHEMICAL SOCIETY (org.) 2010. Chemistry near you: Low-cost experiments for the K-12 classroom. 1. ed. Sao Paulo.