Acids and bases are two chemical groups related to each other. They are two substances of great importance and present in everyday life.
Acids and bases are studied by Inorganic Chemistry, the branch that studies compounds that are not formed by carbon.
Acids and Bases Concepts
The concept of Arrhenius
One of the first concepts of acids and bases developed in the late 19th century by Svante Arrhenius, a Swedish chemist.
According to Arrhenius, acids are substances that in aqueous solution suffer ionization, releasing as cations only H+.
HCl (aq) → H+ (aq) + Cl- (here)
Meanwhile, bases are substances that suffer ionic dissociation, releasing as the only type of anion the OH- (hydroxyl) ions.
NaOH (aq) → Na+ (aq)+OH- (here)
However, Arrhenius' concept for acids and bases was restricted to water.
Also read about: Arrhenius Theory and Neutralization reaction.
The Bronsted-Lowry Concept
The Bronsted-Lowry concept is broader than that of Arrhenius and was introduced in 1923.
According to this new definition, acids are substances capable of donating a proton H
+ to other substances. And bases are substances capable of accepting an H proton+ of other substances.That is, the acid is a proton donor and the base is a proton receptor.
It characterizes a strong acid as one that completely ionizes in water, that is, releases H ions+.
However, the substance may be amphiprotic, that is, capable of behaving like a acid or Bronsted base. Look at the example of water (H2O), an amphiprotic substance:
HNO3(aq) + H2O(l) → NO3- (aq) + H3O+(aq) = Bronsted base, accepted the proton
NH3(aq) + H2O(l) → NH4+(aq) + OH-(aq) = Bronsted acid, donated the proton
Furthermore, substances behave like conjugate pairs. All reactions between an acid and a base of Bronsted involve the transfer of a proton and have two conjugated acid-base pairs. See the example:
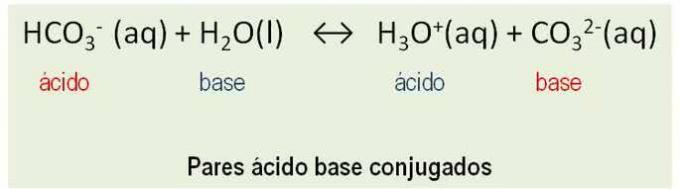
HCO3- and CO32-; H2O and H3O+ are conjugate acid base pairs.
Learn more about:
- Inorganic Functions
- Acid-base indicators
- Titration
Acid nomenclature
To define the nomenclature, acids are divided into two groups:
- Hidracids: acids without oxygen;
- Oxyacids: acids with oxygen.
Hidracids
The nomenclature occurs as follows:
acid + element name + hydric
Examples:
HCl = hydrochloric acid
HI = hydriodic acid
HF = hydrofluoric acid
oxyacids
The nomenclature of oxyacids follows the following rules:
You standard acids of each family (families 14, 15, 16 and 17 of the Periodic Table) follow the general rule:
acid + element name + ic
Examples:
HClO3 = chloric acid
H2ONLY4 = sulfuric acid
H2CO3: carbonic acid
For the other acids that form with the same core element, we name them based on the amount of oxygen, following the following rule:
| Amount of oxygen, in relation to standard acid | Nomenclature |
|---|---|
| + 1 oxygen | Acid + per + element name + ico |
| - 1 oxygen | Acid + element name + bone |
| - 2 oxygens | Acid + hypo + element name + bone |
Examples:
HClO4 (4 oxygen atoms, one more than standard acid): perchloric acid;
HClO2 (2 oxygen atoms, one less than standard acid): chlorous acid;
HClO (1 oxygen atom, two less than standard acid): hypochlorous acid.
You may also be interested in: sulfuric acid
Base Nomenclature
For base nomenclature, the general rule is followed:
Hydroxide + cation name
Example:
NaOH = Sodium hydroxide
However, when the same element forms cations with different charges, the number of the charge of the ion is added to the end of the name, in Roman numerals.
Or, you can add the suffix -oso, to the least charged ion, and the suffix -ico, to the most charged ion.
Example:
Iron
Faith2+ = Fe(OH)2 = Iron II hydroxide or Ferrous hydroxide;
Faith3+ = Fe(OH)3 = Iron III hydroxide or Ferric hydroxide.
Be sure to check entrance exam questions on the subject, with commented resolution, in: Exercises on inorganic functions.