Both aldehydes and ketones have carbonyl as a functional group. The difference is in the position of this carbonyl. As shown below, in aldehydes, the carbonyl appears bonded to a hydrogen, that is, it always comes at the end. On the other hand, in ketones, the carbonyl group is between two carbons, it never comes at the end of the carbon chains:
Aldehydes: Ketones:
OO
║║
H Ç ─ C ─ Ç ─C
The main methods of obtaining compounds belonging to these groups are: hydration of alkynes, ozonolysis of alkenes and oxidation of alcohols. In the case of ketones, there is still a special method, which is the thermal decomposition of organic calcium salts.
See how each of these processes works:
1. Alkynes Hydration:The addition of water molecules takes place in an acidic medium in the presence of the HgSO catalyst4.
Initially, an intermediate compound is formed, an enol, which is transformed into the aldehyde.
If the alkyne that reacts is ethane, we will have the formation of the aldehyde ethanal. But if it's any other alkyne, the corresponding ketones will be formed, following the
Markovnikov's Rule, where the hydrogen in water will add to the carbon in the triple bond that has the most hydrogens attached to it: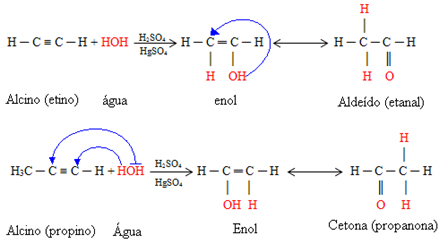
2. Ozonolysis of alkenes: Ozone (O3) is added to the double bond of the alkene, in an oxidation reaction in the presence of water and zinc.
An intermediate compound, ozone, is first formed, which hydrolyzes to the corresponding aldehyde and ketone.
Below we have the ozonolysis of 2-methyl-prop-1-ene, forming propane-2-one and methanol:
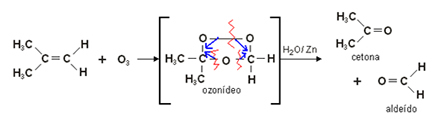
3. Oxidation of Alcohols: Alcohols can undergo oxidation when exposed to an oxidizing agent, such as an aqueous solution of potassium dichromate (K2Cr2O7) or potassium permanganate (KMnO4) in an acidic medium.
If alcohol is primary, partial oxidation generates an aldehyde. But if it's a total oxidation, the aldehyde will turn into a carboxylic acid. If we want to stop at the aldehyde, just carry out this process at a temperature higher than the boiling point of the aldehyde that will be formed. In this way, it evaporates and is distilled through a specific apparatus.

If alcohol is secondary, the product of its oxidation will be a ketone.
Example:
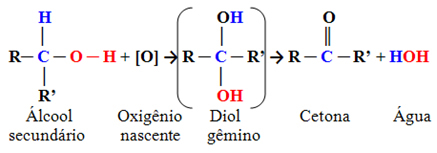
Tertiary alcohols do not undergo oxidation.
4. Specific method of obtaining ketones: Heating of carboxylic acid calcium salts. These salts undergo decomposition, originating, in addition to a ketone, calcium carbonate:
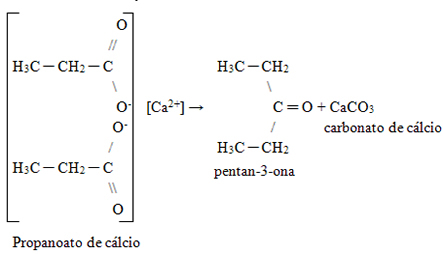
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/metodos-obtencao-aldeidos-cetonas.htm
