The molecules of substances are not all straight, as if they were in a single plane. After all, they are scattered in space and their atoms take on different arrangements or arrangements. Thus, there are different geometric shapes for the molecules of each type of substance.
One of the simplest ways to determine the geometry of a molecule is based on the theory of repulsion of valence shell electron pairs (RPECV). According to this theory, the electron pairs of the central atom function as electronic clouds that repel each other. In this way, they are oriented as far away from each other as possible. Molecular geometry will depend on the number of electronic pairs around the central atom.
This electronic cloud can be composed of electrons that participate in bonds (single, double or triple) and also that do not participate. So we have:
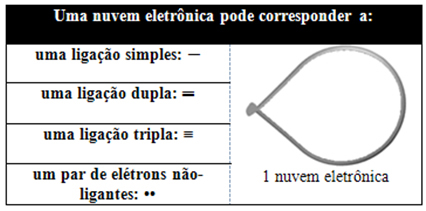
It helps to think of this cloud as a tethered balloon or balloons, with the central atom at the center of them. For example, in a molecule that has only two electron clouds around the central atom, the larger possible distance between them is an angle of 180º and, consequently, the geometry of the molecule will be linear.
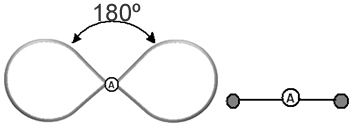
Therefore, we can make a deduction about what the molecular geometry of most molecules will be like if we take into account the number of atoms in the molecules, and the bonds that the central atom makes, checking whether or not it has pairs of electrons.
See the examples below:
- Molecules with 2 atoms: always will be linear
For example:
H ─ H, H ─ Cl, F ─ F, O ═ O, C ≡ O.
- 3-atom molecule: angular or triangular
if the central atom have a pair of non-binding electrons the geometry will be angular, as in the case of the SO molecule2:
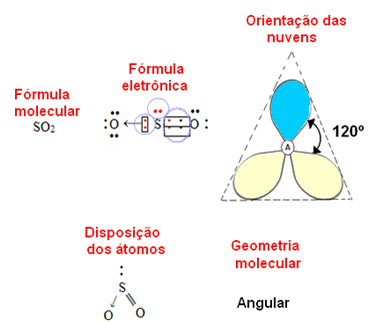
Consider the blue electron cloud to represent pairs of electrons that do not participate in the bonds, and the yellow one to be pairs of electrons that participate in the bonds.
- 4-atom molecule: plane trigonal (or triangular) or pyramidal (or trigonal pyramid)
If the central atom does not have an unpaired (non-bonding) pair of electrons, the molecular geometry is flat trigonal (or triangular). Look at the BF example3:

If the atom has non-binding electron pairs, the geometry of the molecule will be pyramidal (or trigonal pyramid), as in the case of ammonia:

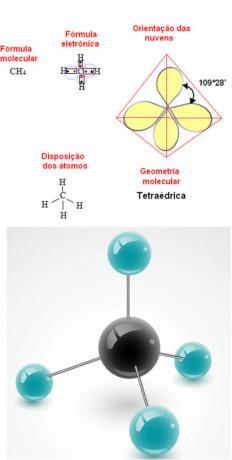
- 5-atom molecule: tetrahedral
Example of Methane Geometry:
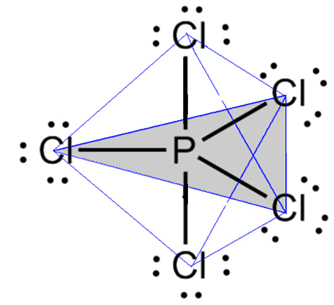
- Molecule with 6 atoms: trigonal bipyramid or triangular pyramid.
An example is phosphorus pentachloride (PCl5), which has some connections between phosphorus and chlorine at an angle of 90º and others of 120º, forming a bipyramid with a triangular base:
- 7-atom molecule: octahedral
Example: sulfur hexafluoride (SF6), whose angles are 90º.

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/determinacao-geometria-das-moleculas.htm
