The different substances that exist in the universe are composed of atoms, ions or molecules. Chemical elements combine through chemical bonds. These links can be:
| Covalent bond | ionic bond | Metal connection |
|---|---|---|
electron sharing |
electron transfer |
Between metal atoms |
Use the questions below to test your knowledge of chemical bonding.
Proposed exercises
question 1
To interpret the properties of different substances, it is necessary to know the bonds between the atoms and the bonds between the respective molecules. In relation to the bond between atoms it can be said that…
(A) between bonded atoms the forces of attraction predominate.
(B) when a bond is formed between atoms, the formed system reaches maximum energy.
(C) the attractions and repulsions of a molecule are not only electrostatic in nature.
(D) between bound atoms there is a balance between electrostatic attractions and repulsions.
Correct alternative: (D) Between bound atoms there is a balance between electrostatic attractions and repulsions.
Atoms are formed by electrical charges and it is the electrical forces between particles that lead to the formation of bonds. Therefore, all chemical bonds are electrostatic in nature.
Atoms have forces of:
- repulsion between nuclei (positive charges);
- repulsion between electrons (negative charges);
- attraction between nuclei and electrons (positive and negative charges).
In all chemical systems, atoms seek to become more stable and this stability is achieved in a chemical bond.
Stability is due to the balance between attraction and repulsion forces, as atoms reach a state of lower energy.
question 2
Correctly match the sentences in column I and the linkage type in column II.
| I | II |
|---|---|
| (A) Between Na atoms | 1. single covalent bond |
| (B) Between Cl atoms | 2. double covalent bond |
| (C) Between atoms of O | 3. Metal connection |
| (D) Between N atoms | 4. ionic bond |
| (E) Between Na and Cl atoms | 5. triple covalent bond |
Reply:
Atoms |
Connection Types |
Representation |
(A) Between Na atoms |
Metallic connection. The atoms of this metal are linked together through metallic bonds and the interaction between positive and negative charges increases the stability of the set. |
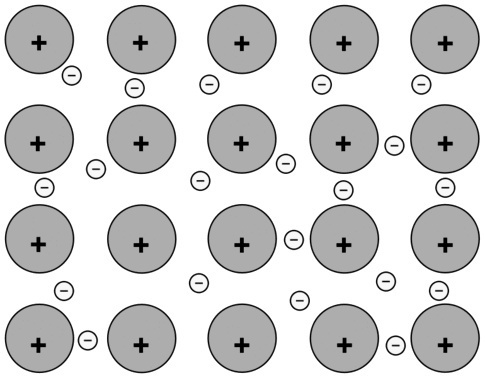 |
(B) Between Cl atoms |
Single covalent bond. Electron sharing and single bond formation occur because there is only one pair of bonding electrons. |
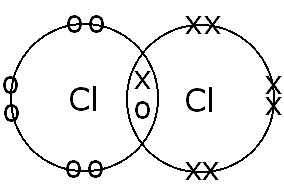 |
(C) Between atoms of O |
Double covalent bond. There are two pairs of bonding electrons. |
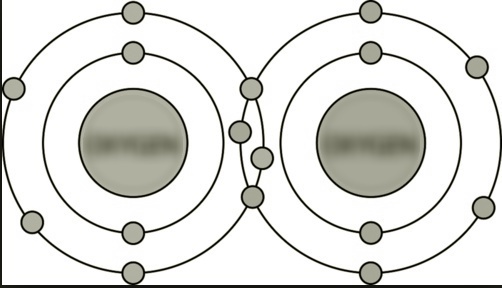 |
(D) Between N atoms |
Triple covalent bond. There are three pairs of bonding electrons. |
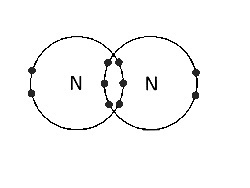 |
(E) Between Na and Cl atoms |
Ionic bond. Established between positive ions (cations) and negative ions (anions) through electron transfer. |
 |
question 3
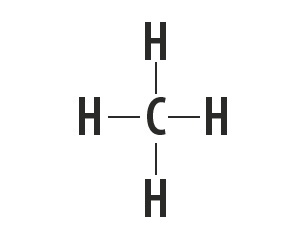
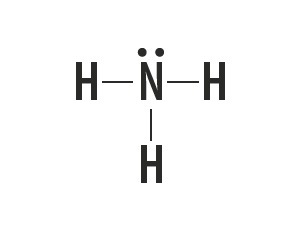
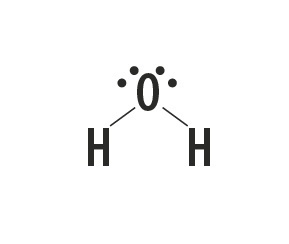
Methane, ammonia, water and hydrogen fluoride are molecular substances whose Lewis structures are represented in the following table.
| Methane, CH4 | Ammonia, NH3 | Water, H2O | hydrogen fluoride, HF |
|---|---|---|---|
 |
 |
 |
 |
Indicates the type of bond established between the atoms that make up these molecules.
Correct answer: Simple covalent bond.
Looking at the periodic table, we see that the elements of substances are not metals.
The type of bond these elements form between them is the covalent bond, as they are sharing electrons.
Atoms of carbon, nitrogen, oxygen and fluorine make up eight electrons in the valence shell because of the number of bonds they make. They then obey the octet rule.
Hydrogen, on the other hand, participates in the formation of molecular substances by sharing a pair of electrons, establishing simple covalent bonds.
See too: Chemical bonds
entrance exam questions
Questions about chemical bonds appear a lot in college entrance exams. See below how the topic can be approached.
question 1
(UEMG) The properties exhibited by a certain material can be explained by the type of chemical bond present between its forming units. In a laboratory analysis, a chemist identified the following properties for a certain material:
- High melting and boiling temperature
- Good electrical conductivity in aqueous solution
- Bad conductor of solid state electricity
From the properties displayed by this material, mark the alternative that indicates the predominant type of connection in it:
(A) metallic
(B) covalent
(C) induced dipole
(D) ionic
Correct alternative: (D) ionic.
A solid material has high melting and boiling temperatures, that is, it would need a lot of energy to change into a liquid or gaseous state.
In the solid state, the material is a poor conductor of electricity because of the organization of atoms that form a well-defined geometry.
In contact with water, ions appear, forming cations and anions, facilitating the passage of electrical current.
The type of bond that makes the material have these properties is the ionic bond.
question 2
(PUC-SP) Analyze the physical properties in the table below:
| Sample | Fusion point | Boiling point | Electrical conductivity at 25°C | Electrical conductivity at 1000°C |
|---|---|---|---|---|
| THE | 801°C | 1413°C | insulating | conductor |
| B | 43°C | 182 °C | insulating | |
| Ç | 1535 °C | 2760°C | conductor | conductor |
| D | 1248°C | 2250 °C | insulating | insulating |
According to the chemical bond models, A, B, C and D can be classified, respectively, as,
(A) ionic compound, metal, molecular substance, metal.
(B) metal, ionic compound, ionic compound, molecular substance.
(C) ionic compound, molecular substance, metal, metal.
(D) molecular substance, ionic compound, ionic compound, metal.
(E) ionic compound, molecular substance, metal, ionic compound.
Correct alternative: (E) ionic compound, molecular substance, metal, ionic compound.
Analyzing the physical states of the samples when they are subjected to the temperatures presented, we have to:
| Sample | Physical state at 25°C | Physical state at 1000°C | Classification of compounds |
| THE | solid | liquid | Ionic |
| B | solid | Molecular | |
| Ç | solid | solid | Metal |
| D | solid | solid | Ionic |
Both compounds A and D are insulators in the solid state (at 25 °C), but when sample A changes to a liquid state it becomes conductive. These are characteristics of ionic compounds.
Solid state ionic compounds do not allow for conductivity because of the way the atoms arrange themselves.
In solution, the ionic compounds turn into ions and allow electricity to be conducted.
It is characteristic of metals that they have good conductivity like sample C.
Molecular compounds are electrically neutral, that is, insulators like sample B.
See too: Metal links
question 3
(Fuvest) Consider the element chlorine forming compounds with, respectively, hydrogen, carbon, sodium and calcium. With which of these elements does chlorine form covalent compounds?
Reply:
| Elements | How the call occurs | bond formed | |
| chlorine | Hydrogen | 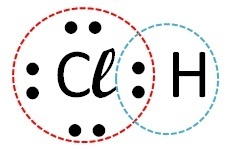 |
Covalent (electron sharing) |
| chlorine | Carbon | 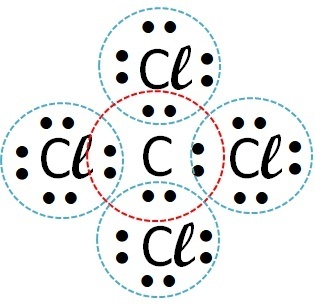 |
Covalent (electron sharing) |
| chlorine | Sodium |  |
Ionic (electron transfer) |
| chlorine | Calcium | 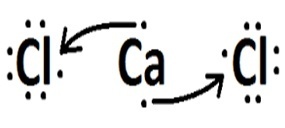 |
Ionic (electron transfer) |
Covalent compounds occur in the interaction of non-metal atoms, non-metals with hydrogen or between two hydrogen atoms.
So the covalent bond occurs with chlorine + hydrogen and chlorine + carbon.
Sodium and calcium are metals and bond to chlorine through an ionic bond.
Enem questions
Enem's approach to the topic may be a little different from what we've seen so far. See how chemical bonds appeared in the 2018 test and learn a little more about this content.
question 1
(Enem/2018) Research shows that nanodevices based on atomic-dimensional movements, induced by light, may have applications in future technologies, replacing micromotors, without the need for components mechanics. An example of light-induced molecular motion can be seen by bending a thin silicon wafer, bonded to an azobenzene polymer and a support material, in two wavelengths, as illustrated in figure. With the application of light, reversible reactions of the polymer chain occur, which promote the observed movement.
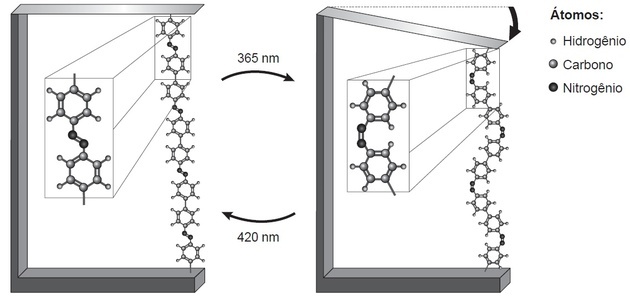
TAKE, H. AND. Nanotechnology of molecules. New Chemistry at School, n. 21, May 2005 (adapted).
The phenomenon of molecular movement, promoted by the incidence of light, arises from (a)
(A) vibrational movement of atoms, which leads to shortening and relaxation of bonds.
(B) isomerization of N=N bonds, the cis form of the polymer being more compact than the trans.
(C) tautomerization of the polymer's monomeric units, which leads to a more compact compound.
(D) resonance between the π electrons of the azo group and those of the aromatic ring that shorten the double bonds.
(E) conformational variation of N=N bonds that results in structures with different surface areas.
Correct alternative: (B) isomerization of N=N bonds, the cis form of the polymer being more compact than the trans.
The movement in the polymer chain causes a longer polymer to be observed on the left and a shorter one on the right.
With the polymer part highlighted, we observe two things:
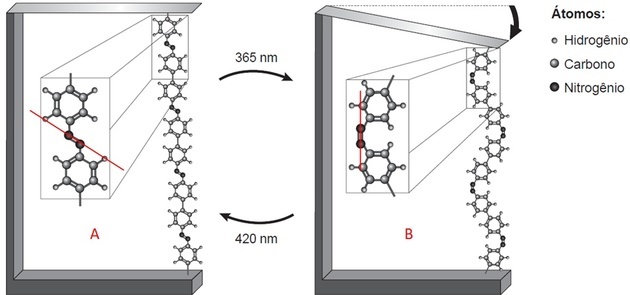
- There are two structures that are linked by a bond between two atoms (which the legend indicates is nitrogen);
- This link is in different positions in each image.
Drawing a line on the image, in A we observe that the structures are above and below the axis, that is, opposite sides. In B, they are on the same side of the drawn line.
Nitrogen makes three bonds to be stable. If it's bound to the structure by a bond, then it's bound to the other nitrogen via a covalent double bond.
Polymer compaction and blade bending occur because the binders are in different positions when isomerism of N=N bonds occurs.
Trans isomerism is observed in A (linkers on opposite sides) and cis in B (linkers in the same plane).
question 2
(Enem/2018) Some solid materials are composed of atoms that interact with each other forming bonds that can be covalent, ionic or metallic. The figure shows the potential energy of binding as a function of the interatomic distance in a crystalline solid. Analyzing this figure, it is observed that, at the temperature of zero kelvin, the equilibrium distance of the bond between the atoms (R0) corresponds to the minimum value of potential energy. Above that temperature, the thermal energy supplied to the atoms increases their kinetic energy and causes they oscillate around an average equilibrium position (filled circles), which is different for each temperature. The connection distance can vary over the entire length of the horizontal lines, identified with the temperature value of T1 the T4 (rising temperatures).
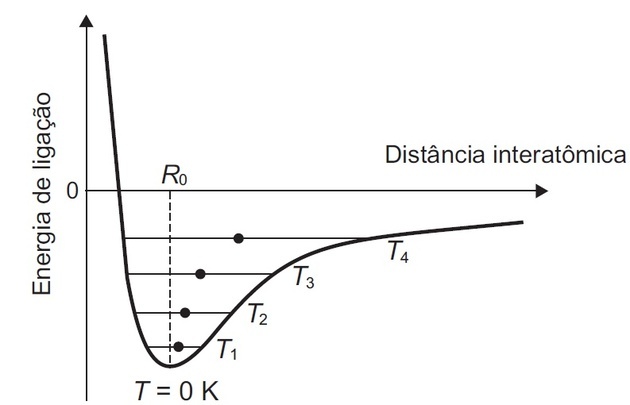
The displacement observed in the average distance reveals the phenomenon of
(A) ionization.
(B) dilation.
(C) dissociation.
(D) breaking of covalent bonds.
(E) formation of metallic bonds.
Correct alternative: (B) dilation.
Atoms have positive and negative charges. Bonds form when they reach a minimum energy by balancing forces (repulsion and attraction) between atoms.
From this we understand that: for a chemical bond to occur there is an ideal distance between the atoms so that they are stable.
The graphic shown shows us that:
- The distance between two atoms (interatomic) decreases until reaching a minimum energy.
- Energy can increase when atoms become so close that the positive charges in their nuclei approach, start to repel each other, and consequently increase energy.
- At temperature T0 of zero Kelvin is the minimum value of potential energy.
- There is an increase in the temperature of T1 to T4 and the energy supplied causes the atoms to oscillate around the equilibrium position (filled circles).
- The oscillation occurs between the curve and the full circle corresponding to each temperature.
As temperature measures the degree of agitation of molecules, the higher the temperature, the more the atom oscillates and the space occupied by it increases.
The highest temperature (T4) indicates that there will be a greater space occupied by that group of atoms and thus, the material expands.
question 3
(Enem/2019) Because they have a complete valence layer, high ionization energy and electronic affinity practically null, it was considered for a long time that the noble gases would not form compounds chemicals. However, in 1962, the reaction between xenon (5s²5p⁶ valence layer) and platinum hexafluoride was successfully carried out and, since then, more new noble gas compounds have been synthesized.
Such compounds demonstrate that one cannot uncritically accept the octet rule, in which it is considered that, in a chemical bond, atoms tend to acquire stability assuming the electronic configuration of gas noble. Among the known compounds, one of the most stable is xenon difluoride, in which two halogen atoms fluorine (2s²2p⁵ valence layer) covalently bond to the noble gas atom to have eight electrons of valence.
When writing the Lewis formula for the aforementioned xenon compound, how many electrons are there in the valence shell in the noble gas atom?
(A) 6
(B) 8
(C) 10
(D) 12
Correct alternative: c) 10.
Fluorine is an element that is part of group 17 of the Periodic Table. Therefore, in its outermost electronic shell there are 7 electrons (2s2 2p5). To acquire stability, according to the octet rule, the atom of this element needs one electron to thus have 8 electrons in the valence shell and assume the electronic configuration of a noble gas.
Xenon, on the other hand, is a noble gas, and, therefore, it already has 8 electrons in the last layer (5s2 5p6).
Note that the name of the compound is xenon difluoride, that is, the compound is made up of two fluorine atoms and one xenon atom, XeF2.
As the statement says, the chemical bond between atoms is of the covalent type, that is, there is the sharing of electrons.
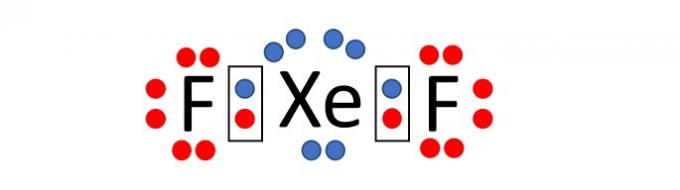
Distributing the electrons around each atom (7 around fluorine and 8 around xenon) we see that the xenon atom, when bonding with two fluorine atoms, has 10 electrons in the fluorine shell. valence.
See too:
- octet rule
- Exercises on electronic distribution
- Exercises on hydrocarbons


