Colloids, colloidal solutions or colloidal system are mixtures that present the appearance of a solution, that is, a homogeneous mixture. But actually they are heterogeneous mixtures.
This is because although it is not clear to the naked eye, the difference in colloidal mixtures can be observed through the use of instruments such as the microscope.
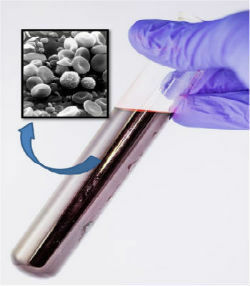 The blood, despite appearing homogeneous, with the use of a microscope we observed that it is made up of several components.
The blood, despite appearing homogeneous, with the use of a microscope we observed that it is made up of several components.
Colloids are present in our daily lives. They are examples of colloids: moisturizing cream, yogurt, milk, blood, dyes and jam.
It is for this reason that some chemical products indicate that they must be shaken before being used. This must be done to unite the colloidal particles.
At the same time, colloidal mixtures do not settle naturally. If we put a colloid in a container, the particles don't settle to the bottom. They also cannot be filtered.
The size of particles present in colloids is between 1 and 100 nanometers (1 nanometer is equivalent to 1 millionth of a millimeter).
Everything outside this range is homogeneous or heterogeneous mixtures.
Homogeneous mixtures are considered true solutions. Its particles are smaller than 1 nanometer. Heterogeneous mixtures have particles larger than 100 nanometers.
Learn more about Chemical Solutions and Separation of Mixtures.
What are your Properties?
The components of colloids are called scattered and dispersant. The amount of dispersant is always higher.
Apparently, they assume a homogeneous mixing characteristic.
One example is egg whites beaten on snow: white in liquid form takes on the role of dispersed component.
The air, which caused the white to turn into a foam, is the dispersing component, as it took more air than the white to achieve this mixture.
In addition, colloids allow light to pass between them, which is not the case with homogeneous mixtures.
If we aim a flashlight with a small beam of light at a colloidal mixture, it is possible to see a beam of light passing through the entire container where it is located. That's what it's called Tyndall effect.
Through the same experiment, it is also possible to detect the random movement of particles in the mixture. This is called Brownian movement.
In short, the properties of colloidal systems they are:
- The phases of the mixture are not easily distinguishable;
- The particle size range is 1 and 100 nanometers;
- Tyndall Effect;
- Presence of dispersed particles and dispersants;
- They do not settle naturally, nor can they be filtered;
- Brownian movement.
Types of Colloids
Colloids are classified according to the physical state of dispersed and dispersing particles.
The types of colloids are: aerosol, emulsion, foam, gel and sol (those that look like a solution). Learn more about each of them:
Aerosol
Dispersed component: Solid or Liquid
Dispersant Component: gas
Examples: Smoke, fog, cloud, spray
Emulsion
Dispersed component: Liquid
Dispersant Component: Liquid or Solid
Examples: Mayonnaise, butter, cheese, ice cream
Foam
Dispersed component: gas
Dispersant Component: Liquid or Solid
Examples: Whipped cream, white in snow, shaving foam, popcorn
Gel
Dispersed component: Liquid
Dispersant Component: solid
Examples: Gelatin, silica gel, toothpaste
Sun
Dispersed component: solid
Dispersant Component: Liquid or Solid
Examples:Pearl, Ruby, Blood
To learn more, learn about a method for separating colloidal mixtures, the Centrifugation.