One buffer solution it is a mixture used to prevent the pH or pOH of the medium from changing when strong acids or strong bases are added.
There are two types of buffer solution:
1. Mixture of weak acid with its conjugate base;
2. Mixture of weak base with its conjugate acid.
Let's look at examples of each and how they work when a small amount of strong acid or base is added to the medium:
1. Mixture of weak acid with its conjugate base:
To form such a solution, the weak acid is mixed with a salt of the same anion as the acid.
For example, consider a buffer solution consisting of acetic acid (H3CCOOH(here)) and sodium acetate (H3CCOONa(s)). See that both have the acetate anion: (H3CCOO-(here)). The concentration of these ions is practically due to the dissociation of the salt, which is large. The acid ionization is small.

Now notice what happens in the following addition possibilities:
- Addition of a small amount of strong acid:
The addition of a strong acid increases the concentration of the hydronium ion, H
3O+1, and since acetic acid is a weak acid, the acetate anion has a high affinity for the proton (H+) hydronium. In this way, they react and more acetic acid is formed:
As a result, the pH of the medium practically does not change. However, if more and more strong acid is added, the time will come when all the acetate anion will be consumed and the buffering effect will cease.
- Addition of a small amount of strong base:
The addition of a strong base increases the concentration of OH ions-. But these ions are neutralized by the H ions3O+1 released in the ionization of acetic acid:

With this reaction, the concentration of H ions3O+1(here) will decrease and there will be a shift in the equilibrium towards increasing the acid ionization and, therefore, the pH variation of the solution will be very small. The concentration of H ions3O+1(here) it will be practically constant.
Do not stop now... There's more after the advertising ;)
In this case, there is also a limit capacity of the buffer. Therefore, if we add more and more base, the balance of acid ionization will be shifted more and more towards its ionization, until all the acid is consumed.
2. Mixture of a weak base with its conjugate acid:
This type of buffer solution is made up of a weak base and a salt solution that contain the same cation as the base.
For example, consider a buffer solution formed by magnesium hydroxide, MgOH2(aq) (weak base) and magnesium chloride, MgCl2(s) (salt). Both contain the cation magnesium (Mg2+(here)). The magnesium ions present in the medium are practically all derived from the dissociation of the salt, as the dissociation of the base is weak:
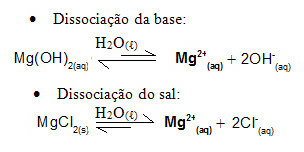
- Addition of a small amount of strong acid:
In this case, the H ions3O+1 coming from the addition of strong acid will be neutralized by OH ions-, coming from weak base dissociation. This will shift the base dissociation balance to the right.
Thus, the pH variation (if any) will be very small, because the concentration of OH ions- remains constant. The buffering effect will cease when the entire base is dissociated.
- Addition of a small amount of strong base:
The added strong base undergoes dissociation releasing OH ions-. Since magnesium hydroxide is a weak base, the magnesium released on dissociation from the salt will have a greater tendency to react with the OH-:

Therefore, the increase in OH ions- is offset by the proportional increase in Mg(OH)2(aq). As a result, the pH does not undergo major changes.
This effect ends when all the magnesium cation has been consumed.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "What is a buffer solution?"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/o-que-uma-solucao-tampao.htm. Accessed on June 28, 2021.
Chemistry

Test your knowledge and learn more with this list of solved exercises on chemical balances. Through this material, you will be able to better understand how to work equilibrium constants (Kp, Kc and Ki), equilibrium shift, pH and pOH, as well as equilibrium in so-called buffer solutions.
Solution, Solid Solution. Liquid solution, gaseous solution, solutions formed by gas and liquid, solutions formed by liquids.