Nitrogen Functions are one of the 4 functional groups of organic compounds. The compounds that belong to this function are formed by nitrogen, so they are called nitrogen compounds. The main ones are amines, amides, nitriles and nitro compounds.
Amines
At amines are organic compounds that can be found in solid, liquid or gaseous states. They are produced by the decomposition of animals and can also be found in compounds extracted from plants.
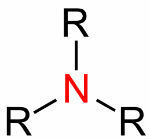
General Formula of Amines
They are derived from aryl or alkyl in connection with nitrogen. Aryl and alkyl replace hydrogen atoms. According to this replacement, they can be classified as follows:
- Primary: when only one hydrogen is replaced (R-NH2). Example: methanamine.
- Secondary: when two hydrogens are replaced (R1R2NH). Example: dimethaneamine.
- Tertiary: when three hydrogens are replaced (R1R2R3N). Example: trimethaneamine.
Amines are used in the manufacture of dyes, medicines and soaps.
The nomenclature of amines is formed as follows:
- replacing the hydrocarbon suffix “o” with the word amine.
- indicating the position of the nitrogen.
- indicating the type of binding an, en or in.
amides
At amides are organic compounds that can be found in solid or liquid states. They are derived from acyl bound to nitrogen and produced in the laboratory.
The molecular formula of the amides is CONH2, which is represented as follows:
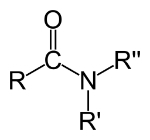
General Formula of Amides
Amides are classified according to the number of acyls bound to nitrogen that have:
- Primary: when it has only one acyl group R-CO)NH2.
- Secondary: when they have two acyl groups (R-CO)2NH.
- Tertiary: when they have three acyl groups (R-CO)3N.
As for the number of amide groups present, the classification is as follows:
Diamides, when there are two groups of amides, and triamides, when there are three groups of amides, etc.
Its applications in everyday life: manufacturing of personal care cosmetics (shower gel) and cleaning (detergent) products, among others.
The name of the amides is formed by the prefix, which indicates the number of carbons. Next, the suffix “oico” for hydrocarbon is replaced by the word amide.
Nitrocompounds
Nitro compounds are organic compounds found in a liquid state that do not dissolve in water because they are dense and highly reactive. The general formula for nitro compounds is AT THE2.
The application of nitro compounds is comprehensive. They are used as an explosive, as a solvent and also in the manufacture of ointments and tools.
The name of nitro compounds is formed by joining the name of the main chain with the word nitro.
Nitriles
Nitriles, also called cyanides, are organic compounds found in a solid state and are soluble in water. The general formula of nitriles is R — C ≡ N.
These compounds are used in the manufacture of rubber, dyes, fertilizers and plastics.
The name of nitriles is formed by joining the name of the hydrocarbon with the word nitrile.
Read too Oxygenated Functions.
Exercises
1. (Cesgranrio-RJ) In early 1993, the newspapers reported that when a person falls in love, the organism synthesizes a substance - ethylphenylamine, responsible for the characteristic excitation of that state.
The classification and chemical character of this amine are, respectively:
a) primary amine – acid.
b) primary amine – basic.
c) secondary amine – neutral.
d) secondary amine – acid.
e) secondary amine – basic.
Alternative e: secondary amine – basic.
2. (UnB-DF) Acetoaminophen is a substance that has analgesic and antipyretic properties. It is marketed under the name Tylenol and its formula is outlined below:
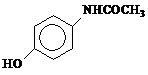
Tick the correct alternatives regarding acetoaminophen.
1. It belongs to the class of phenols;
2. It also contains the amide function;
3. Has formula C8H9AT THE2;
4. It belongs to the class of aromatic substances due to the presence of the benzene ring.
All alternatives are correct