THE isomerism it is a phenomenon observed when different compounds are formed by equal amounts of the same types of atoms.
Therefore, we can find chemical substances with the same molecular formula and different structural formulas or spatial arrangements.
This phenomenon is widely observed in Organic Chemistry, which studies carbon compounds. As each atom of this element can make 4 chemical bonds, it is possible to observe different combinations.
As the change in the arrangement of atoms gives rise to new substances, consequently, they have different physical and chemical properties.
When we say which compounds are isomers means that they have equal parts, as the word is a combination of two terms of Greek origin: isos, means "really", and mere, which is “parts”.
The two major groups of isomerism are plane and space (stereoisomerism).
THE flat isomerism it can be visualized by the flat structural formula of the compounds and has five classes: position, function, chain, compensation and tautomery.
THE space isomerism it is observed by the orientation of the compounds and is subdivided into geometric (cis-trans) and optical.
The concept of isomerism was introduced in 1830 by the Swedish scientist Jacob Berzelius.
Examples of isomers
THE chain isomerism it occurs when the same set of atoms connect to form structures of the same functional group, but with different chains.
Example: the molecular formula C4H10 of a hydrocarbon can be straight or branched.

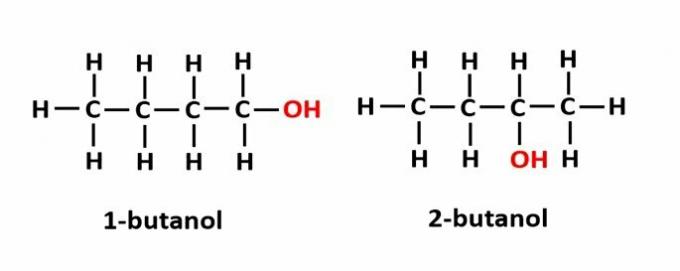
THE position isomerism occurs when compounds are formed by the same functional groups, but they are at different positions in the chain.
Example: The molecular formula C4H9OH corresponds to two types of alcohol.
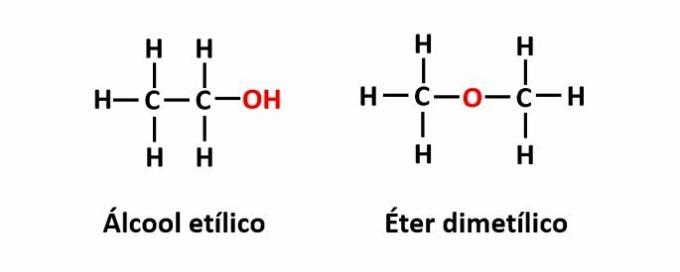
THE function isomerism occurs when the same molecular formula corresponds to two compounds with different functional groups.
Example: The molecular formula C2H6O corresponds to two isomers that have the alcohol (-OH) and ether (-O-) functions.
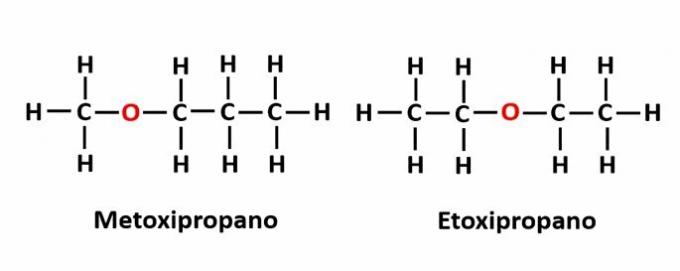
THE compensation isomerism it occurs when there is a heteroatom in the chain and it occupies different positions. This is a particular case of position isomerism.
Example: The molecular formula C4H10The indicates the presence of an oxygen atom in the chain, but its position can change and form different compounds.
THE dynamic isomerism, also called tautomery, occurs when two compounds with different functions are in the same solution in dynamic equilibrium. This is a particular case of function isomerism.
Example: The molecular formula C2H4O corresponds to compounds with the aldehyde and enol functions.
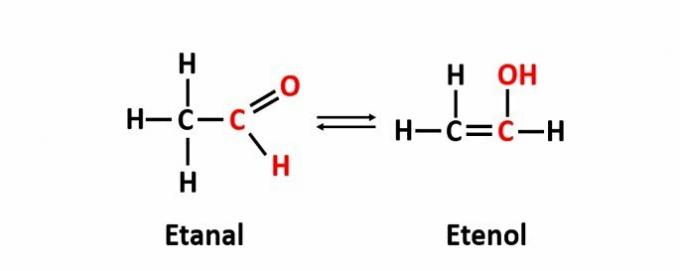
Learn more about flat isomerism.
THE geometric isomerism, also called isomery cis-trans, occurs when the presence of a double bond or cyclic structure causes equal ligands to be on the same side of the plane (cis) or on opposite sides (trans).
Example: the chlorine atoms in compounds of molecular formula C2H2Cl2 they may have two spatial structures.
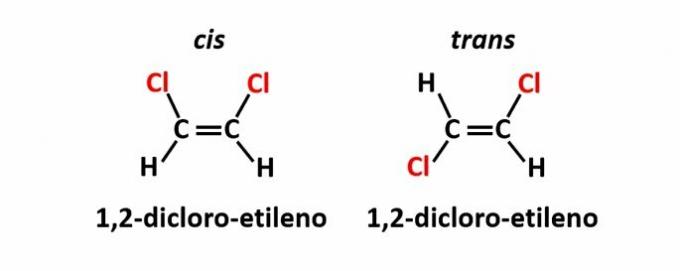
THE optical isomerism occurs when, when deflecting the polarized light emitted on the structure, the compounds manage to deflect the beam luminous to the left, if it is a levorotatory (l) isomer, or to the right, when it is a dextrorotatory isomer (d).
Example: This type of isomerism occurs with lactic acid. Note below that the spectral images of the isomers do not overlap, hence they are called enantiomers.
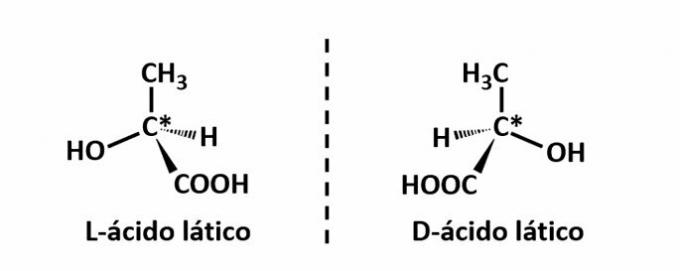
The symbol C* indicates the presence of a chiral carbon in this structure, ie a carbon atom with 4 different linkers.
Learn more about isomerism with the contents:
- Types of isomerism
- space isomer
- geometric isomer
- optical isomer
- Exercises on plane isomerism