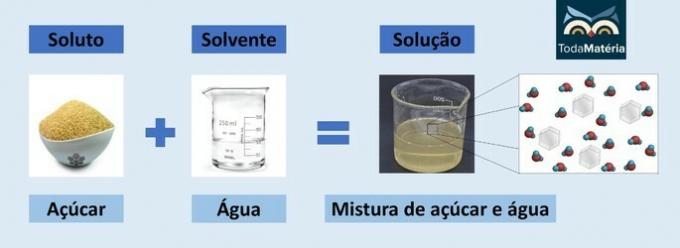
Molarity (M) is the relationship between the solute matter (n) and the volume of a solution (V), that is, M = n/V.
Since solute matter is given in mol and volume is given in liters, the unit of measure for molarity is mol/L.
It is also known by the names molar concentration, concentration in mol/L or concentration in quantity of matter.
Formula
The molarity formula is:
M = m/M.V
Where,
M = molarity
m = mass of solute (given in g)
M = molar mass (given in g/mol)
V = volume (given in l)
This formula arises from the fact that solute matter is usually given in grams. Thus, to obtain the solute matter (n) we must divide the solute mass by its molar mass.
How to calculate
The calculation of the molarity of a solution can be done in the following ways:
1) For example, if we know that there are 0.4 mol of a solute in 100 ml of solution, just substitute the values given in the formula M = n/V, ie,
M = 0.4/0.1
M = 4 mol/L
2) Now if, for example, we know that 200 ml of a sodium hydroxide solution has 0.5 mol/L, we still need to know what its mass is.
First, we have to add up the mass of each of the elements that make up sodium hydroxide: sodium, oxygen and hydrogen. These values can be obtained from the periodic table (sodium 23, oxygen 16 and hydrogen 1, 23 + 16 + 1 = 40).
Next, we can use the formula M = m/M. V, that is,
M = m/M.V
0.5 = m/40.0.2
m = 0.5.40.0.2
m = 4 g
And Molality?
THE molality (W) or molal concentration is also the result of the amount of matter in the solute per volume of the solution.
What makes molarity different from molality is that molality is used to calculate higher values, always in kilograms (kg).
Read too:
- Titration
- Colligative Properties
- Density
Exercises
1. (Mack-2004) The molarities of Cu ions2+ and NO 1-3, in a 0.5 molar solution of Cu (NO3)2, are, respectively:
a) 0.5M and 0.5M.
b) 0.5M and 1.0M.
c) 1.0M and 1.0M.
d) 2.0M and 0.5M.
e) 0.5M and 1.5M.
Alternative b) 0.5M and 1.0M.
2. (PUC - PR-2007) A student needed to prepare an aqueous solution of 0.50 mol/L NaCl to set up a marine aquarium, with a maximum capacity of 80 L.
So, mixed 25 L of NaCl(here) 0.40 mol/L, which had been stored in a gallon, with 35 L of solution from another deactivated aquarium, whose NaCl concentration was 0.75 mol/L.
The molarity of NaCl of the solution obtained in this way was:
a) higher than expected and to correct it he should add 12 L of pure water.
b) lower than expected and to correct it he should add 5 L of pure water.
c) the expected value.
d) higher than expected and to correct it he should add 12 L of another 0.40 mol/L NaCl solution..
e) below expectations and to correct it he should add 12 L of another 0.40 mol/L NaCl solution..
Alternative a) higher than expected and to correct it he should add 12 L of pure water.
3. (UFF-1999) Potassium permanganate can be used as a germicide in the treatment of burns. It is a shiny solid and is commonly used as a common reagent in laboratories.
Consider dissolving 0.395 g of this salt in enough water in an acidic medium to produce 250 mL of solution. The molarity of the resulting solution is:
a) 0.01 M
b) 0.02 M
c) 0.03 M
d) 0.04M
e) 0.05 M
Alternative a) 0.01 M
For more questions about concentrating solutions, check out the list we've prepared.: Exercises on Common Concentration.