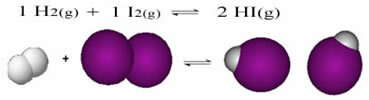
Endothermic and Exothermic Reactions are quantities that measure the amount of heat (energy) absorbed and released during chemical reactions. They are studied by thermochemistry.
What's the difference between them?
endothermic reaction is the one in which there is energy absorption. In this process, energy is produced and independently maintained.
Birds and mammals have the ability to maintain stable body temperature. For this reason they are called endothermic animals, popularly "warm-blooded animals".
Exothermic Reaction is the one in which there is energy release. In this process, energy production only persists through the continuous supply of energy.
Changing physical states, in that order: gas, liquid, and solid, is an example of an exothermic reaction. Each of them occurs as energy is released, that is, when there is less heat.
Note that when we reverse this order (solid, liquid and gas), energy is produced (more heat). In this case, the reaction is endothermic.
Everyday Examples
Body fat acts as fuel in our body. The one that is not burned is absorbed by it. As this is an absorption process, it is an example of an endothermic reaction.
When placing a pan on the fire to prepare food, we are, in turn, facing an exothermic process. That's because the heat that is released will transform this food so that it can be consumed.
And what is Enthalpy?
enthalpy it is the energy that exists in all substances and that is altered as a result of endothermic and exothermic reactions.
As it is not possible to calculate the enthalpy, the calculation of its variation was established.
Thus, by comparing the standard enthalpy (temperature of 25º C under atmospheric pressure of 1atm), it would be possible to calculate the enthalpy variation.
According to the Hess' Law, the final enthalpy minus the initial enthalpy (ΔH = Hf - Hi) results in this data.
If the endothermic reaction absorbs energy, this means that the enthalpy of the (final) reactant is lower than that of the (initial) product. Therefore, the enthalpy change is positive (ΔH > 0).
In turn, if the exothermic reaction releases energy, it means that the enthalpy (energy) of the reactant is greater than that of the product. Therefore, the enthalpy variation is negative (ΔH
Read too:
- Chemical reactions
- Chemical balance
- Combustion