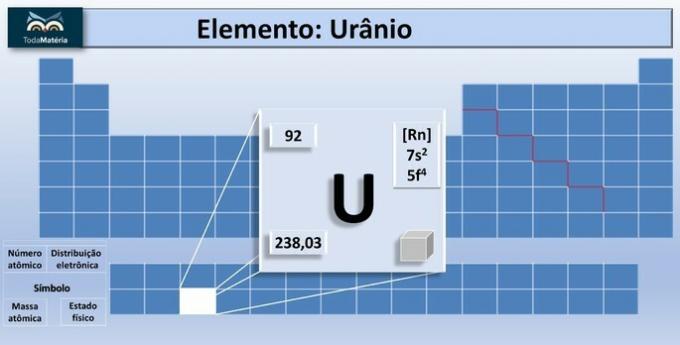
Uranium is a chemical element on the Periodic Table represented by the symbol U, whose atomic number is 92 and belongs to the actinide family.
It is the element with the heaviest atomic nucleus in nature.
The best known isotopes of uranium are: 234U, 235Huh 238U.
Due to the radioactivity of this metal, its main application is in generating nuclear energy through the fission of its core. Furthermore, uranium is used in rock dating and nuclear weaponry.
Uranium Characteristics
- It's a radioactive element.
- Dense metal with high hardness.
- Ductile and malleable.
- Its color is silvery gray.
- It is found in abundance in the solid state.
- Its atom is highly unstable and the 92 protons in the nucleus can be disintegrated and form other chemical elements.
Uranium Properties
Physical properties
| Density | 18.95 g/cm3 |
|---|---|
| Fusion point | 1135 °C |
| Boiling point | 4131 °C |
| Toughness | 6.0 (Mohs scale) |
Chemical properties
| Classification | Internal transition metal |
|---|---|
| electronegativity | 1,7 |
| Ionization energy | 6.194 eV |
| Oxidation States | +3, +4, +5 ,+6 |
Where is Uranium found?
In nature, uranium is mainly found in the form of ores. To explore the reserves of this metal, the present content of the element and the availability of technology to carry out the extraction and exploitation are studied.
Uranium ores
Due to its ease of reaction with oxygen in the air, uranium is usually found in the form of oxides.
| Ore | Composition |
|---|---|
| pitchblende | U3O8 |
| Uraninite | ou2 |
uranium in the world
Uranium can be found in several parts of the world, being characterized as a common ore for being present in most rocks.
The largest uranium reserves are found in the following countries: Australia, Kazakhstan, Russia, South Africa, Canada, the United States and Brazil.
Uranium in Brazil
Although not all of the Brazilian territory has been prospected, Brazil occupied the seventh position in the world ranking of uranium reserves.
The two main reserves are Caetité (BA) and Santa Quitéria (CE).
Uranium isotopes
| Isotope | relative abundance | half life time | radioactive activity |
|---|---|---|---|
| Uranium-238 | 99,27 % | 4,510,000,000 years | 12,455 Bq.g-1 |
| Uranium-235 | 0,72 % | 713,000,000 years | 80.011 Bq.g-1 |
| Uranium-234 | 0,006 % | 247,000 years | 231 x 106 Bq.g-1 |
Because it is the same chemical element, all isotopes have 92 protons in the nucleus and, consequently, the same chemical properties.
Although the three isotopes have radioactivity, the radioactive activity is different for each of them. Only uranium-235 is a fissile material and, therefore, useful in the production of nuclear energy.
Uranium radioactive series
Uranium isotopes can undergo radioactive decay and generate other chemical elements. What happens is a chain reaction until a stable element is formed and transformations cease.
In the following example, the radioactive decay of uranium-235 ends with lead-207 being the last element in the series.
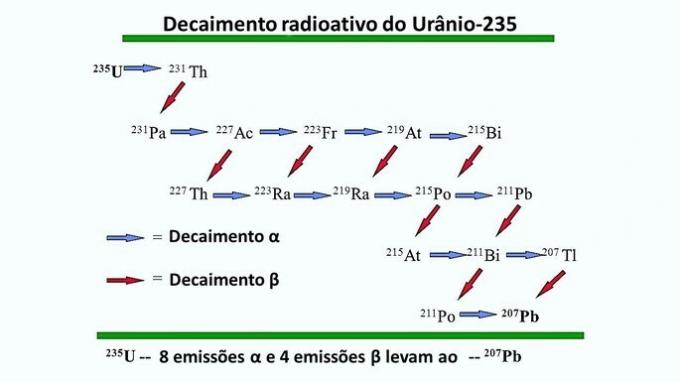
This process is important in determining the Earth's age by measuring the amount of lead, the last element in the radioactive series, in certain uranium-containing rocks.
History of Uranium
Its discovery took place in 1789 by the German chemist Martin Klaproth, who gave it its name in honor of the planet Uranus, also discovered around this period.
In 1841, uranium was isolated for the first time by the French chemist Eugène-Melchior Péligot through a reduction reaction of uranium tetrachloride (UCl).4) using potassium.
Only in 1896, the French scientist Henri Becquerel discovered that this element had radioactivity when carrying out experiments with uranium salts.
Uranium Applications
Nuclear energy

Uranium is an alternative energy source for existing fuels.
The use of this element to diversify the energy matrix is due to the increase in the price of oil and gas, in addition to the environmental concern with the release of CO2 in the atmosphere and the greenhouse effect.
Energy production occurs through the fission of the uranium-235 core. A chain reaction is produced in a controlled manner and from the numerous transformations that the atom undergoes, there is the release of energy that moves a steam generation system.
The water is transformed into steam when receiving energy in the form of heat and causes the system's turbines to move and generate electrical energy.
Transformation of Uranium into Energy
The energy released by uranium comes from nuclear fission. When a larger nucleus is broken, a large amount of energy is released in the formation of smaller nuclei.
In this process, there is a chain reaction that starts with a neutron hitting a large nucleus and breaks it into two smaller nuclei. The neutrons released in this reaction will cause the fission of other nuclei.
When hit by a neutron, uranium-235 split into two smaller nuclei and released 3 neutrons.
The energy released in this reaction is 2.1010 kJ/mol. In ethanol combustion, the energy released is 98 kJ/mol. Given this, we can see the magnitude of this process, whose energy produced is practically a trillion times greater than a combustion reaction.
Nuclear energy in Brazil
Brazil has two nuclear power plants that use enriched uranium. They are located in the municipality of Angra dos Reis (RJ).
According to Eletronuclear, the company that operates thermonuclear plants in Brazil, Angra 1 has capacity to generate 657 megawatts of electricity, while Angra 2 can generate 1,350 megawatts electric.
radiometric dating

In radiometric dating, radioactive emissions are measured according to the element generated in the radioactive decay.
Knowing the half-life of the isotope, it is possible to determine the age of the material by calculating how much time it took for the found product to form.
Uranium-238 and uranium-235 isotopes are used to estimate the age of igneous rocks and other types of radiometric dating.
Atomic bomb
At Second World War the first atomic bomb was used, which contained the element uranium.
With the uranium-235 isotope a chain reaction started from the fission of the nucleus, which in a fraction of a second, generated an explosion due to the extremely powerful amount of energy released.
Check out more texts on the subject:
- Manhattan Project
- Hydrogen bomb
- Nuclear fusion
- Nuclear waste