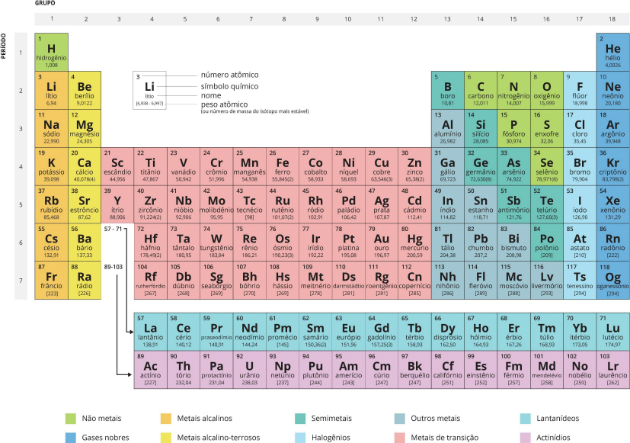
The periodic table is a model that groups all known chemical elements and presents some of their characteristics. Currently, the periodic table has 118 chemical elements.
Evolution of the Periodic Table
The periodic table model we know today was proposed by the Russian chemist Dmitri Mendeleev (1834-1907), in the year 1869.
The fundamental purpose of creating a table was to facilitate the classification, organization and grouping of chemical elements according to their properties.
Many scholars were already trying to organize this information and, therefore, many previous models were presented.
From Ancient Greece came the first attempts to organize the known elements. empedocles was a Greek philosopher who spoke of the existence of four "elements": water, fire, earth and air.
Posteriorly, Aristotle he made the first organization of these elements and associated them with some "properties" such as wet, dry, hot and cold.
Antoine Lavoisier (1743-1794) noted that through the electrolysis, the water decomposed into hydrogen and oxygen. He then classified the substances found in elementary because he could not divide them into simpler substances.
He identified some of the first chemical elements and, in 1789, compiled a list of 33 elements divided into sets. of simple, metallic, non-metallic and earthy substances, but it could not establish a property that differentiated.
Johann W. Döbereiner (1780-1849) was one of the first to observe an order to organize the chemical elements. As by the early 19th century approximate atomic mass values for some elements had been established, he organized groups of three elements with similar properties.

The classification model proposed by Döbereiner drew a lot of attention from the scientific community at the time. He suggested an organization based on triads, that is, elements were grouped into trios according to their similar properties.
THE atomic mass of the central element was the average of the masses of the other two elements. For example, sodium had an approximate mass value that corresponded to the average of the masses of lithium and potassium. However, many elements could not be grouped in this way.
Alexandre-Emile B. of Chancourtois (1820-1886), French geologist, organized 16 chemical elements in ascending order of atomic mass. For this, he used a model known as the Telluric Screw.
In the model proposed by Chancourtois, information is distributed at the base, in a cylinder shape, vertically aligning elements with similar properties.

John Newlands (1837-1898) also played a key role. He created the law of octaves for chemical elements.
His observations showed that, arranging the elements in ascending order of atomic mass, for every eight elements the properties were repeated, thus establishing a periodic relationship.

Newlands' work was still restricted, as this law even applied to calcium. However, his thinking was a precursor to Mendeleev's ideas.
Julius Lothar Meyer (1830-1895), based mainly on the physical properties of the elements, made a new distribution according to atomic masses.
He observed that between consecutive elements, the difference in masses was constant and concluded that there was a relationship between atomic mass and properties of a group.
Through the study proposed by Meyer, it was possible to prove the existence of periodicity, that is, the occurrence of similar properties at regular intervals.
Dmitri Mendeleev (1834-1907), in 1869, while in Russia, he had the same idea as Meyer, who was studying in Germany. He more meticulously organized a periodic table, where the 63 known chemical elements were arranged in columns based on their atomic masses.

In addition, it left empty spaces in the table for elements that were not yet known. Mendeleev was able to describe some information on the missing elements based on the sequence he drew up.
Mendeleev's Work was the most complete so far, as he organized the elements according to their properties, gathered a large amount of information in a simple way and found that new elements would be discovered, leaving spaces to insert them in the table.
Until then, nothing was known about the constitution of the atoms, but the organization proposed by Meyer-Mendeleiev gave rise to numerous investigations to justify the periodicity of the elements and constitutes the basis of the current Periodic Table.
Henry Moseley (1887-1915), in 1913, made important discoveries, establishing the concept of atomic number. With the development of studies to explain the structure of atoms, a new step was taken for the organization of chemical elements.
From his experiments, he assigned whole numbers to each element and, later, it was found to correspond to the number of protons in the nucleus of the atom.
Moseley reorganized the table proposed by Mendeleiev according to the atomic numbers, eliminating some flaws in the previous table and established the concept of periodicity as follows:
Many physical and chemical properties of elements periodically vary in the sequence of atomic numbers.
In fact, all the proposed models, in some way, contributed to the discoveries about chemical elements and their classifications.
In addition, they were fundamental for arriving at the current model of periodic table that presents 118 chemical elements.
Complete and Updated Periodic Table
The periodic table receives this name in relation to periodicity, that is, the elements are organized in such a way that their properties are repeated in a regular way.
Meet the Periodic table complete and updated:
Read more about related topics:
- Chemical elements
- Periodic properties
Exercises on the Periodic Table
1) Establish the correspondence between the scientists, in column I, and the contributions made by each to the organization of the chemical elements in the Periodic Table, in column II.
| Column I | Column II |
|---|---|
| a) Aristotle | 1) His scheme did not foresee the possibility of other chemical elements being discovered. |
| b) Antoine Lavoisier | 2) Organized the chemical elements according to their atomic number. |
| c) Johann Döbereiner | 3) Organized the “elements”: fire, water, earth and air, associating them with “properties”. |
| d) John Newlands | 4) Identified some of the first chemical elements. |
| e) Dmitri Mendeleev | 5) Left unfilled spaces in the Periodic Table for elements that could be discovered. |
| f) Henry Moseley | 6) He observed that certain groups of three elements shared similar properties. |
Reply:
1-d; 2-f; 3-a; 4-b; 5-e; 6-c.
Newlands' work did not foresee the possibility of other chemical elements being discovered, because it was based on organizing the previously known chemical elements. It was Mendeleev who thought further and observed this possibility.
From Moseley's work, the chemical elements were organized by atomic number and we arrived at the current periodic table.
In Ancient Greece, the first attempts to organize the elements started with Aristotle, but scientists at the time believed that there was only air, fire, earth and water.
Lavoisier was the one who detected the first chemical elements, such as when decomposing water into hydrogen and oxygen.
Döbereiner proposed one of the first organizations of chemical elements, grouped into triads.
2) As the chemical elements were being discovered, scientists were studying their properties and found the existence of certain similarities in the properties of some of them. This fact led them to think of a way to organize the elements according to these properties.
Please classify the following statements as true or false:
2.1 The known chemical elements are organized according to their properties in the Periodic Table.
2.2 The current periodic table contains 118 chemical elements.
2.3 The current periodic table is organized in ascending order of atomic mass.
Answer: V, V, F.
The periodic table groups the elements of the 118 known chemical elements, some natural and some artificial, according to their properties and in ascending order of atomic number.
Check entrance exam questions with a commented resolution in Exercises on the Periodic Table and unpublished questions on the subject in Exercises on Organizing the Periodic Table.