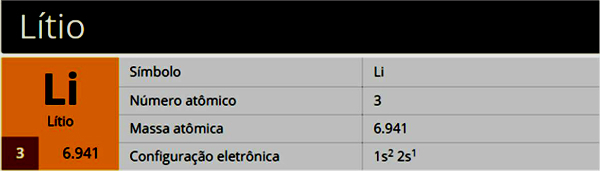
Lithium is a chemical element with the symbol Li, atomic number 3, atomic mass 7, belonging to group 1 (Family 1A), being an alkali metal.
Its name derives from the Greek lithos, which means stone, as the element is found in rocks.
Features
As it is a very reactive element, it is not found in isolation in nature. In its pure form it oxidizes easily in the presence of air or water.
It is found in the minerals spodumene, lepidolite and petalite. In addition to rocks, it also occurs in salt and thermal waters. In industrial environments, it is obtained through the electrolysis of lithium chloride.
It is characterized by being a soft, soft and silver colored metal. In contact with air, it acquires a gray color, so it is common to store it in mineral oil.
Among other features are:
- Good conductor of electricity;
- Extremely reactive;
- Very flammable;
- Metal of lower density is even less dense than water.
Learn more, read also:
- Chemical elements
- Periodic table
applications
Lithium has different types of use, from industrial applications to drug production:
- Manufacture of batteries from lithium ions;
- Participates in the functioning of cardiac pacemakers;
- Lithium carbonate is used in the formulation of psychiatric medications, such as against bipolar depression and tranquilizers;
- Participates in the formation of metal alloys;
- Production of lubricants for machines that work subjected to high temperatures;
- Manufacture of heat resistant ceramics and glass;
- Industrial drying systems in the form of lithium chloride or bromide.
Also read about other chemical elements:
- Barium
- Phosphor
- Helium