Some compounds do not need to have eight electrons in the valence shell to achieve stability, so they are considered exceptions to the Octet Rule. Learn now why some elements escape the obligation of having an octet of electrons in the last shell.
Beryllium (Be)
It is an exception to the Octet Rule because it is able to form compounds with two single bonds, so it stabilizes with only four electrons in the valence shell. 
Since hydrogen (H) needs to give up two electrons to make the bond (H - Well - H), the Beryllium (Be) atom shares its electrons and achieves stability.
Aluminum (Al)
It is an exception to the Octet Rule because it achieves stability with six electrons in the valence shell. The Aluminum atom tends to donate its electrons and so can form three single bonds with other atoms: 
In this case, Aluminum (Al) formed three bonds with three Fluorine (F) atoms.
Boron (B)
It forms molecular substances with three single bonds. 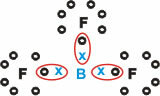
Note that Boron (B) tends to donate its electrons to Fluorine (F) atoms, which obeys the Octet Rule, requiring eight electrons in the valence shell. As boron gives up its electrons, fluorine stabilizes with the octet formed.
By Líria Alves
Graduated in Chemistry
Brazil School Team
Do not stop now... There's more after the advertising ;)
See more!
Octet Theory
General chemistry - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Exceptions to the Octet Rule"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/excecoes-regra-octeto.htm. Accessed on June 27, 2021.