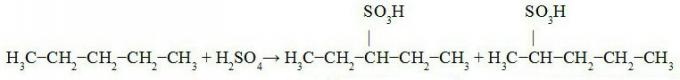Everyone knows about the danger of Cesium 137, but what exactly does this chemical compound consist of? Cesium is a radioactive isotope resulting from the nuclear fission of uranium or plutonium, the problem is that this isotope becomes disintegrates and gives rise to Barium 137m, hence the number 137, from this event onwards, the compound starts to emit gamma radiation.
Gamma rays are extremely harmful to health because they have a great penetration power, they invade the body's cells and can even lead to death. The biggest accident caused by Cesium 137 occurred in the city of Goiânia on September 13, 1987, and resulted in the death of more than 400 people.
Do not stop now... There's more after the advertising ;)
Barium 137 disintegrates into a highly toxic, blue, phosphorescent powder. The National Nuclear Energy Commission (Cnem) is responsible for inspecting radioactive devices and the dispose of them when they no longer serve to be used, so that no new accidents occur nuclear weapons.
The health consequences of radioactive contamination are: diseases such as cancer, hypertension and various disorders.
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Definition of Cesium 137"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/definicao-cesio-137.htm. Accessed on June 27, 2021.