THE optical isomerism is a kind of space isomerism, or stereoisomerism, which studies the behavior of substances when subjected to a beam of polarized light. To better understand, read the text "Polarized Light”.
As in all types of isomerism, optical isomers have the same molecular formula but are differentiated by their optical activity.
For example, consider the lactic acid molecule shown below. Since it is not symmetrical, it can give rise to two types of lactic acids:
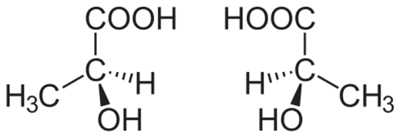
When we submit these two molecules to a polarized light beam, we notice that one of them deflects the polarized light beam to the right, being called right-handed (acid d-lactic); and the other deviates to the left, called levogyro (acid ℓ-lactic). Dextrorotatory lactic acid is obtained by the action of bacteria in the meat extract, and levorotary lactic acid from the fermentation of sucrose by the Bacillus acidi levolactiti.
So these two compounds are optical isomers.
One way to check whether a molecule of a particular compound performs optical activity is to see if the molecule has any
asymmetric carbon (C*), that is, it has 4 different ligands.Do not stop now... There's more after the advertising ;)
Note that this occurs in the structure of lactic acid, and this type of carbon is called chiral, which originates from a Greek word meaning “hand”. So, like our hand, optical stereoisomers are the mirror image of each other, being called because of this the enantiomers.
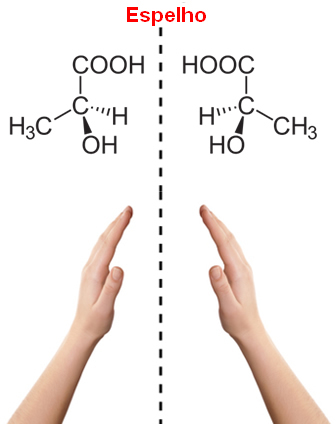
In addition, it should also be noted that the structures of their molecules do not overlap, because the structure of a given molecule and its image overlap without change, so they are the same molecule and not optical isomers.
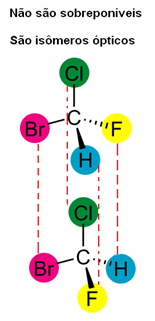
However, it is only through the use of a polarimeter that it is possible to know with certainty whether an optical isomer is right-handed or left-handed.
These two compounds, despite being chemically and physically the same, have totally different properties. A mixture of them is optically inactive, that is, it does not deviate from the polarized light plane and is called racemic mixture.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Optical Isomerism"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/isomeria-Optica.htm. Accessed on June 27, 2021.