When two solutions are mixed, whether they are different or not, it is necessary to first analyze whether or not there is a reaction between them. For example, if we mix a solution of water with sugar (aqueous solution of sucrose) with a solution of water with salt (brine), we will obtain a mixing of solutions without chemical reactions.
The same happens if we mix two solutions of sodium chloride (NaCl), with different concentrations. In this case, there will also be no reaction. We can then define this example as a mixing of solutions of the same solute, without any chemical reaction, where the first example is a mixing of solutions of different solutes, without chemical reaction.
In both cases, the chemical makeup of the components of the solutions will not change., however, some quantitative aspects will have to be recalculated.
To understand how we could determine the molar concentration (Molarity) and the common concentration of a mixture of solutions without a reaction, let us see the resolution of the two cases mentioned:
1st) Mixture of solutions of the same solute, without the occurrence of chemical reaction:

Imagine that we mix two sodium chloride solutions, one with a concentration of 2.0 g/L in 60.0 mL of solution and the other with 2.5 g/L in 80 mL of solution volume.
Since no reaction occurs, both mass and volume are just the sum of the initial masses and volumes:
m (solution) = m1 (NaCl) + m2 (NaCl)
m1 (NaCl) = v. C m2 (NaCl) = v. Ç
m1 (NaCl) = 0.06L. 2.0g/L m2 (NaCl) = 0.08L. 2.5 g/L
m1 (NaCl) =0.1 gm2 (NaCl) =0.2 g
m (solution) = 0.1 g + 0.2 g
m (solution) =0.3 g
v (solution) = v1 (NaCl) + v2 (NaCl)
v (solution) = (60 + 80) mL
v (solution) =140 mL = 0.14 L
Concentration can then be obtained using these data:
C (solution) = m (solution)
v (solution)
C (solution) = 0.3 g
0.14L
C (solution)≈ 2.14 g/L
2nd) Mixture of different solute solutions, without the occurrence of chemical reaction:
Take for example the mixture between 500 ml of an aqueous solution of sucrose (C12H22O11) which initially had a concentration of 18.0 g/L, with 1 L of a salt water solution (aqueous solution of sodium chloride – NaCl) with a concentration of 100.0 g/L.
Do not stop now... There's more after the advertising ;)
After mixing, what was the molarity, common concentration, mass and volume of the solution resulting from mixing?
Since there was no chemical reaction, the masses of C12H22O11 and NaCl remain unchanged. And initial mass values can be achieved by a simple rule of three using reaction concentrations.
18.0 g 1 L
m (C12H22O11) 0.5L
m (C12H22O11) = 9.0 g
m (NaCl) 100.0 g
Mass can also be achieved by the formula:
m = v. Ç
m (C12H22O11) = 0.5 L. 18g/L
m (C12H22O11) = 9.0 g
m (NaCl) = 1 L. 100.0 g/L
m (NaCl) = 100.0 g
Thus, the total mass of the solution is the sum of the two masses:
m (solution) = m (C12H22O11) + m (NaCl)
m (solution) = 109.0 g
The volume is simply the sum of the initial volumes, so we have:
v (final solution) = v (C12H22O11) + v (NaCl)
v (final solution) = (0.5 + 1)L
v (final solution) = 1.5L
The final concentration is achieved by separately calculating the concentrations of each of the solutes. Since they do not react with each other and their masses do not change, we can use the following concentration formula:
C = m
v
initial = minitial final c = mFinal
vinitial vFinal
minitial = mFinal
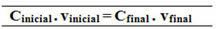
C (C12H22O11) =?
Çinitial. vinitial = CFinal. vFinal
18.0 g/L. 0.5 L = CFinal .1.5 L
C (C12H22O11) Final = 6.0 g/L
C(NaCl)=?
Çinitial. vinitial = CFinal. vFinal
100.0 g/L. 1 L = Cfinal .1.5 L
C (NaCl)Final = 66.67 g/L
The relationship made for this formula of the common concentration can also be done to calculate the molarity (Mi. vi = Mf. vf) and for the concentration in mass by mass (Title - Ti. vi = Tf. vf).
By Jennifer Fogaça
Graduated in Chemistry