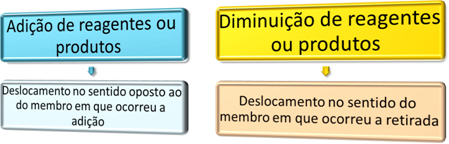
Hess' Law allows us to calculate the enthalpy variation, which is the amount of energy present in substances after undergoing chemical reactions. This is because it is not possible to measure the enthalpy itself, but its variation.
Hess' Law underlies the study of Thermochemistry.
This Law was experimentally developed by Germain Henry Hess, who established:
The enthalpy change (ΔH) in a chemical reaction depends only on the initial and final states of the reaction, regardless of the number of reactions.
How can Hess' Law be calculated?
The enthalpy change can be calculated by subtracting the initial enthalpy (before the reaction) from the final enthalpy (after the reaction):
ΔH = Hf - Hi
Another way to calculate it is through the sum of the enthalpies in each of the intermediate reactions. Regardless of the number and type of reactions.
ΔH = ΔH1 + ΔH2
Since this calculation only considers the initial and final values, it is concluded that the intermediate energy does not influence the result of its variation.
This is a particular case of Principle of Energy Conservation, a First Law of Thermodynamics.
You should also know that Hess' Law can be calculated as a mathematical equation. To do this, you can perform the following actions:
- Reverse the chemical reaction, in which case the ΔH sign must also be reversed;
- Multiply the equation, the value of ΔH must also be multiplied;
- Divide the equation, the value of ΔH must also be divided.
know more about enthalpy.
Enthalpy diagram
Hess' Law can also be visualized through energy diagrams:
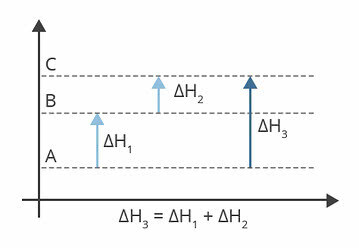
The diagram above shows the enthalpy levels. In this case, the reactions suffered are endothermic, that is, there is energy absorption.
ΔH1 is the enthalpy change that happens from A to B. Suppose it is 122 kj.
ΔH2 is the enthalpy change that happens from B to C. Suppose it is 224 kj.
ΔH3 is the enthalpy change that happens from A to C.
So, it matters to us to know the value of ΔH3, as it corresponds to the change in enthalpy of the reaction from A to C.
We can find the value of ΔH3, from the sum of the enthalpy in each of the reactions:
ΔH3 = ΔH1 + ΔH2
ΔH3 = 122 kj + 224 kj
ΔH3 = 346 kj
Or ΔH = Hf - Hi
ΔH = 346 kj – 122 kj
ΔH = 224 kj
Entrance Exam: Solved step by step
1. (Fuvest-SP) Based on enthalpy variations associated with the following reactions:
N2(g) + 2 O2(g) → 2 NO2(g) ∆H1 = +67.6 kJ
N2(g) + 2 O2(g) → N2O4(g) ∆H2 = +9.6 kJ
It can be predicted that the enthalpy variation associated with the NO dimerization reaction2 will be equal to:
2 NO2(g) → 1 N2O4(g)
a) -58.0 kJ b) +58.0 kJ c) -77.2 kJ d) +77.2 kJ e) +648 kJ
Resolution:
Step 1: Invert the first equation. That's because NO2(g) it needs to move to the reactants side, according to the global equation. Remember that when reversing the reaction, ∆H1 also reverses the sign, changing it to negative.
The second equation is conserved.
2 NO2(g) → N2(g) + 2 O2(g) ∆H1 = - 67.6 kJ
N2(g) + 2 O2(g) → N2O4(g) ∆H2 = +9.6 kJ
Step 2: Note that N2(g) appears in products and reagents and the same happens with 2 mol of O2(g).
2 NO2(g) → N2(g)+ 2 O2(g)∆H1 = - 67.6 kJ
N2(g) + 2 O2(g) → N2O4(g) ∆H2 = +9.6 kJ
Thus, they can be canceled resulting in the following equation:
2 NO2(g) → N2O4(g).
Step 3: You can see that we have arrived at the global equation. Now we must add the equations.
∆H = ∆H1 + ∆H2
∆H = - 67.6 kJ + 9.6 kJ
∆H = -58 kJ ⇒ Alternative A
From the negative value of ∆H we also know that it is an exothermic reaction, with the release of heat.
Learn more, read also:
- thermochemistry
- Exercises on Thermochemistry
- Endothermic and Exothermic Reactions
- Second Law of Thermodynamics
Exercises
1. (UDESC-2012) Methane gas can be used as fuel, as shown in equation 1:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
Using the thermochemical equations below, which you deem necessary, and the concepts of Hess' Law, obtain the enthalpy value of equation 1.
Ç(s) + H2O(g) → CO(g) + H2(g) ΔH = 131.3 kJ mol-1
CO(g) + ½ the2(g) → CO2(g) ΔH = – 283.0 kJ mol-1
H2(g) + ½ the2(g) → H2O(g) ΔH = – 241.8 kJ mol-1
Ç(s) + 2H2(g) → CH4(g) ΔH = – 74.8 kJ mol-1
The enthalpy value of equation 1, in kJ, is:
a) - 704.6
b) - 725.4
c) - 802.3
d) - 524.8
e) - 110.5
c) – 802.3
2. (UNEMAT-2009) Hess' Law is of fundamental importance in the study of Thermochemistry and can be stated as “the variation of enthalpy in a chemical reaction depends only on the initial and final states of the reaction". One of the consequences of Hess' Law is that thermochemical equations can be treated algebraically.
Given the equations:
Ç (graphite) + O2(g) → CO2(g) ΔH1 = -393.3 kj
Ç (Diamond) + O2(g) → CO2(g) ΔH2 = -395.2 kj
Based on the above information, calculate the enthalpy change of graphite carbon to diamond carbon and tick the correct alternative.
a) -788.5 kj
b) +1.9 kj
c) +788.5 kj
d) -1.9 kj
e) +98.1 kj
b) +1.9 kj