Colligative properties involve studies on the physical properties of solutions, more precisely of a solvent in the presence of a solute.
Although not to our knowledge, the colligative properties are widely used in industrial processes and even in various everyday situations.
Related to these properties are the physical constants, for example, the boiling or melting temperature of certain substances.
As an example, we can cite the automobile industry process, such as adding additives to car radiators. This explains why in colder places, the water in the radiator does not freeze.
Processes carried out with foods, such as salting meat or even foods saturated with sugar, prevent the deterioration and proliferation of organisms.
In addition, the desalination of water (salt removal) as well as the spreading of salt on snow in places where winter is very harsh, corroborate the importance of knowing the colligative effects on solutions.
Want to learn more about concepts related to colligative properties? Read the articles:
- Physical States of Water
- Melting Point and Boiling Point
- Water Desalination
- Separation of Mixtures
Solvent and Solute
First of all, we must pay attention to the concepts of solvent and solute, both components of a solution:
- Solvent: substance that dissolves.
- Solute: dissolved substance.
As an example, we can think of a solution of water with salt, where water represents the solvent and salt, the solute.
Want to know more? Read too Solubility.
Colligative Effects: Types of Colligative Properties
Colligative effects are associated with phenomena that occur with the solutes and solvents of a solution, being classified as:
Tonometric Effect
Tonoscopy, also called tonometry, is a phenomenon that is observed when the decrease in the maximum vapor pressure of a liquid (solvent).
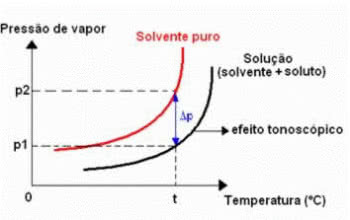
Tonometric Effect Chart
This occurs through the dissolution of a non-volatile solute. Therefore, the solute decreases the solvent's evaporation capacity.
This type of colligative effect can be calculated by the following expression:
ΔP =p0 - P
Where,
ΔP: absolute lowering of the maximum vapor pressure to the solution
P0: maximum vapor pressure of pure liquid, at temperature t
P: maximum vapor pressure of the solution, at temperature t
Ebuliometric Effect
Ebullioscopy, also called ebulliometrics, is a phenomenon that contributes to the increase in temperature variation of a liquid during the boiling process.
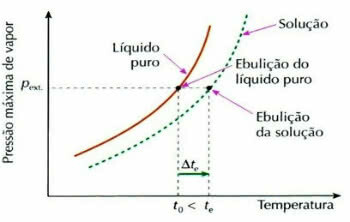
Ebuliometric Effect Graph
This occurs through the dissolution of a non-volatile solute, for example, when we add sugar to the water that is about to boil, the boiling temperature of the liquid increases.
The so-called ebulliometric (or ebullioscopic) effect is calculated by the following expression:
tand = tand – t0
Where,
tand: rise in the boiling temperature of the solution
tand: initial boiling temperature of the solution
t0: boiling temperature of pure liquid
Cryometric Effect
Cryoscopy, also called cryometry, is a process in which the freezing temperature decreaseof a solution.
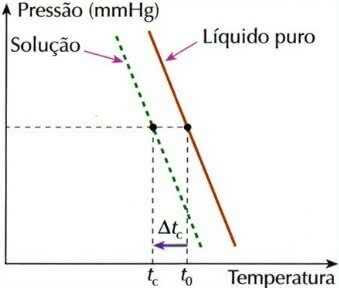
Cryometric Effect Graph
This is because when a non-volatile solute is dissolved in a liquid, the freezing temperature of the liquid decreases.
An example of cryoscopy is the antifreeze additives that are placed in car radiators in places where the temperature is very low. This process prevents the freezing of water, helping the useful life of car engines.
In addition, the salt spread on the streets in places where winter is very cold, prevents the accumulation of ice on the roads.
To calculate this colligative effect, the following formula is used:
tç = t0 – tç
Where,
tç: lowering the freezing temperature of the solution
t0: freezing temperature of pure solvent
tç: initial freezing temperature of the solvent in the solution
Check out an experiment on this property at: Chemistry Experiments
Raoult's Law
The so-called “Raoult's Law” was proposed by the French chemist François-Marie Raoult (1830-1901).
He studied the colligative effects (tonometric, ebuliometric and cryometric), helping in the studies of the molecular masses of chemical substances.
By studying the phenomena associated with the melting and boiling of water, he came to the conclusion that: when dissolving 1 mole of any non-volatile and non-ionic solute in 1 kg of solvent always have the same tonometric, ebuliometric or cryometric.
Thus, Raoult's Law can be expressed as follows:
“In a non-volatile and non-ionic solute solution, the colligative effect is proportional to the molality of the solution.”.
It can be expressed as follows:
Psolution = xsolvent. Ppure solvent
Also read about the Mol Number and Molar Mass.
osmometry
Osmometry is a type of colligative property that is related to osmotic pressure of solutions.
Remember that osmosis is a physicochemical process that involves the passage of water from a less concentrated (hypotonic) medium to a more concentrated (hypertonic) medium.
This takes place through a semi-permeable membrane, which only allows the passage of water.
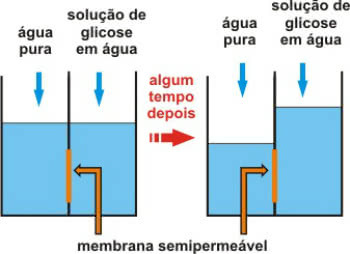
Action of the semipermeable membrane after a while
The call osmotic pressure it is the pressure that allows the water to move. In other words, it is the pressure exerted on the solution, which prevents its dilution by passing the pure solvent through the semi-permeable membrane.
Therefore, osmometry is the study and measurement of osmotic pressure in solutions.
Note that in the water desalination technique (salt removal) the process called reverse osmosis.
Read more about Osmosis.
Laws of Osmometry
The Dutch physicist and chemist Jacobus Henricus Van’t Hoff (1852-1911) was responsible for postulating two laws associated with osmometry.
The first law can be expressed as follows:
“At constant temperature, the osmotic pressure is directly proportional to the molarity of the solution.”
In the second law postulated by him, we have the following statement:
“At constant molarity, the osmotic pressure is directly proportional to the absolute temperature of the solution.”
Therefore, to calculate the osmotic pressure of molecular and diluted solutions, the formula is used:
π = MRT
being,
π: solution osmotic pressure (atm)
M: solution molarity (mol/L)
R: universal constant of perfect gases = 0.082 atm. L/mol. K
T: absolute temperature of the solution (K)
Read too Molarity.
Entrance Exam Exercises with Feedback
1. Comparing two pans, simultaneously on two identical burners on the same stove, it is observed that the pressure of gases in boiling water in a closed pressure cooker is greater than that in boiling water in a pressure cooker open.
In this situation, and if they contain exactly the same amounts of all the ingredients, we can to state that, compared to what happens in the open pan, the cooking time in the pressure cooker closed will be:
a) lower, as the boiling temperature will be lower.
b) lower, as the boiling temperature will be higher.
c) smaller, as the boiling temperature does not vary with pressure.
d) equal, as the boiling temperature is independent of pressure.
e) higher, as the pressure will be higher.
Alternative b
2. (UFRN) In severe winter places, it is customary to add a certain amount of ethylene glycol to the water in car radiators. The use of a solution instead of water as a coolant is because the solution has:
a) lower heat of fusion.
b) lower freezing point.
c) higher freezing point.
d) higher heat of fusion.
Alternative b
3. (Vunesp) One of the ways to heal wounds, according to popular belief, is to put sugar or coffee powder on them. The colligative property that best explains the removal of fluid, by the procedure described, favoring healing, is studied by:
a) osmometry.
b) cryoscopy.
c) endoscopy.
d) tonoscopy.
e) ebulliometrics.
Alternative to
4. (UFMG) In a freezer, there are five ways that contain different liquids, to make ice and lemon popsicles. If the molds are placed in the freezer at the same time and are initially at the same temperature, the mold containing 500 ml of: will be frozen first
a) pure water.
b) solution, in water, containing 50 ml of lemon juice.
c) solution, in water, containing 100 ml of lemon juice.
d) solution, in water, containing 50 ml of lemon juice and 50 g of sugar.
e) solution, in water, containing 100 ml of lemon juice and 50 g of sugar.
Alternative to
5. (Cesgranrio-RJ) The melting point of a substance x was determined, finding a value lower than the tabulated for this substance. This could mean that:
a) the amount of substance used in the determination was less than necessary.
b) the amount of substance used in the determination was greater than necessary.
c) a part of the substance has not melted.
d) the substance contains impurities.
e) the substance is 100% pure.
Alternative