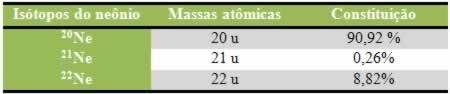
Atomic models emerged from the need to explain the structure of atoms. When new evidence about the constitution of atoms was presented, a new atomic model tried to clarify the findings.
The Greek philosophers Democritus and Leucipo in the V century; Ç. called the atom, from the Greek ατoμoν, the indivisible particle and the smallest part of matter.
Although the concept of the atom is old, the development of atomic theories dates back to the 19th and 20th century. Therefore, the main atomic models developed to understand the nature of matter were:
- Dalton's Atomic Model (1803) — "Billiard Ball Model"
- Thomson Atomic Model (1898) — "Rain Pudding Model"
- Rutherford's Atomic Model (1911) — "Nuclear Model"
- Bohr's Atomic Model (1913) — "Planetary Model"
- Quantum Atomic Model (1926) — "Electronic Cloud Model"
Dalton's Atomic Model
The first recognized attempt to describe atoms came from the English scientist John Dalton (1766-1844) in a model that became popularly known as the “billiard ball”.
Dalton's Atom (1803): massive, indivisible and indestructible sphere.

According to Dalton:
- All substances are formed by atoms;
- The atoms of a chemical element are identical in size and characteristics, whereas those of different chemical elements are different;
- Substances are the result of a chemical reaction, which consists of the recombination of atoms.
Negative points: Since electrons were not yet known when Dalton formulated his theory, these particles, which we now know to be part of atoms, were not considered.
Learn more about Dalton's atomic model.
Thomson Atomic Model
Joseph John Thomson (1856-1940) was responsible for discovering the existence of electrons, particles endowed with a negative charge and that are part of atoms. This discovery overturned Dalton's atomic theory, that the atom is indivisible, but formed by even smaller particles and, therefore, it became known as “raisin pudding”.
Thomson's Atom (1898): positively charged sphere with fixed electrons.

According to Thomson:
- The atom is electrically neutral;
- Electrons attach themselves to a positively charged surface;
- There is a repulsion between electrons distributed in atoms.
Negative points: Although Thomson took into account the existence of electrons, the atom is not a positive sphere, but rather endowed with positively charged particles, the protons, identified in 1886 by scientist Eugene Goldstein and later confirmed by Ernest Rutherford.
Learn more about Thomson atomic model.
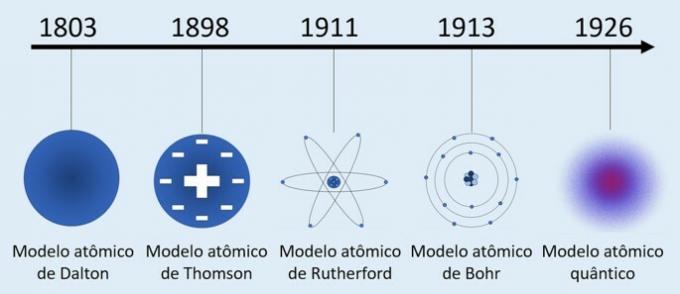
Rutherford Atomic Model
Through his experiments Ernest Rutherford (1871-1937) was able to demonstrate that the atom was not an indivisible particle as believed, but that it was formed by smaller particles.
Rutherford's Atom (1911): positively charged nucleus and electrons are located around it in the electrosphere.
According to Rutherford:
- The atom has a central region with a high concentration of positive charge;
- The mass of an atom is concentrated in its central region;
- Electrons are lighter and are located around the nucleus, a region that contains many empty spaces.
Negative points: The atomic nucleus does not only have positively charged particles, but there are also other subatomic particles, the neutrons, discovered by James Chadwick in 1932. Furthermore, the model proposed by Rutherford did not explain the emission of light by atoms.
Learn more about Rutherford atomic model.
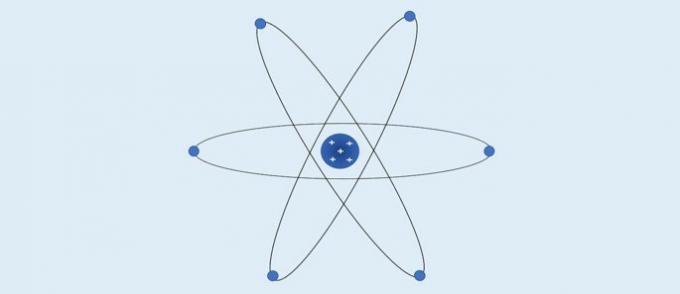
Bohr's atomic model
Seeking to explain why the elements emit characteristic colors when exposed to some conditions and based on the Rutherford's atomic model, Niels Bohr (1885-1962) proposed an atomic theory that explained the emission of light in certain frequencies.
Bohr's Atom (1913): electrons move in fixed circular layers around the nucleus.
According to Bohr:
- Electrons move in the layers around the nucleus;
- The layers around the core have specific energy values;
- To go to a more external level, the electron must absorb energy. Upon returning to a layer closer to the nucleus, the electron releases energy.
Negative points: It cannot be said that electrons travel around the nucleus in fixed positions like the planets around the Sun.
Learn more about Bohr atomic model.
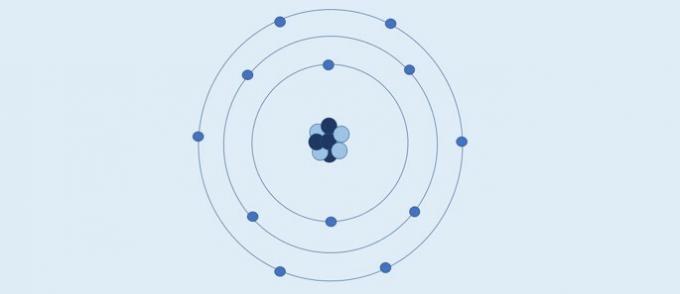
Quantum Atomic Model
Many scientists have contributed to the development of quantum mechanics, which tries to explain the "more real" structure of a atom by the combination of several studies and, therefore, it is the most complex.
Quantum Atom (1926): the nucleus is made up of protons (positive charge) and neutrons (zero charge), and electrons (negative charge) form an electronic cloud around the nucleus.
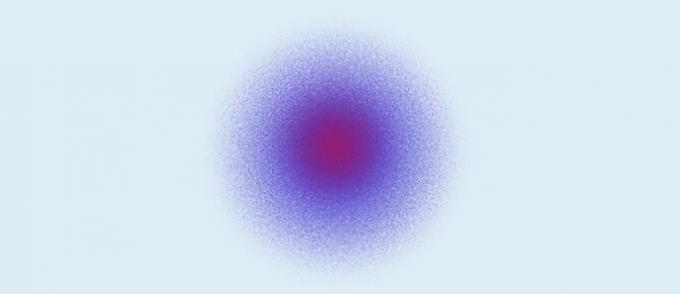
According to the quantum atomic model:
- The nucleus is made up of protons and neutrons. Since only protons have a charge, the nucleus is positively charged;
- Electrons form an electronic cloud around the nucleus;
- Electrons move in orbitals, in three-dimensional space;
- The exact position of an electron cannot be defined. What is done are calculations that determine the probability of the region that an electron will be in a given time.
You quantum numbers have the function of locating the electrons. Are they:
O principal quantum number (n) represents the energy levels, that is, the electronic layers of an atom.
O secondary quantum number (l) indicates the energy sublevels, that is, the energy sublevel to which the electron belongs.
O magnetic quantum number (m) is the one that indicates the orbit where the electrons meet.
Learn more about atomic models and test your knowledge with exercises on atomic models.