Thermochemistry is the part of chemistry that studies the amount of heat (energy) involved in chemical reactions.
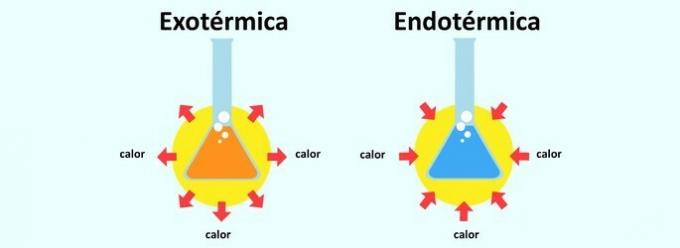
When a reaction releases heat, it is classified as exothermic. The absorption of heat in a reaction causes it to be endothermic.
Thermochemistry also studies the transfer of energy in some physical phenomena, such as changes in the states of matter.
Thermochemistry and heat
In chemical reactions, energy can be absorbed or released. This heat transfer is from the body with the highest temperature to the one with the lowest temperature.
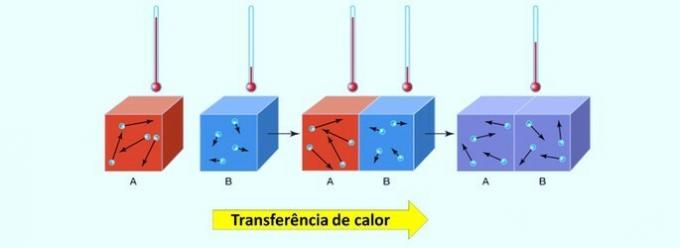
It is worth remembering that heat, also called heat energy, is a concept that determines the exchange of thermal energy between two bodies. O thermal balance is established when the two materials reach the same temperature.
Endothermic and Exothermic Reactions
it's called endothermic reaction the reaction in which heat is absorbed. In this way, a body absorbs heat from the environment in which it is inserted. That's why the endothermic reaction causes a feeling of cooling.
Example: When rubbing alcohol on the arm, the arm absorbs the heat of this substance. But, when blowing on the arm after having passed alcohol, we feel a little chill, a sensation that is the result of the endothermic reaction.
already the exothermic reaction it's the reverse. It is the release of heat and thus the sensation is warming.
Example: In a camp, people stand by a fire so that the heat released by the flames warms those around them.
Thermal exchanges also happen in the changes of physical state. It turns out that, in the change from solid to liquid and from liquid to gas, the process is endothermic. Conversely, the change from gas to liquid and from liquid to solid is exothermic.
enthalpy
Enthalpy (H) is the energy exchanged in the energy absorption and release reactions, respectively, endothermic and exothermic.
There is no device capable of measuring enthalpy. For this reason, its variation (ΔH) is measured, which is done considering the enthalpy of the reactant (initial energy) and the enthalpy of the product (final energy).
The most recurrent types of enthalpy are:
| Formation enthalpy | Absorbed or released energy needed to form 1 mole of a substance. |
|---|---|
| Combustion enthalpy | Released energy that results in the burning of 1 mole of substance. |
| Link enthalpy | Energy absorbed in the breaking of 1 mole of chemical bond, in the gaseous state. |
While enthalpy measures energy, entropy measures the degree of disorder of chemical reactions.
Hess' Law
Germain Henry Hess established that:
The enthalpy change (ΔH) in a chemical reaction depends only on the initial and final states of the reaction, regardless of the number of reactions.
The variation of energy, according to Hess' Law, is established through the following formula:
ΔH = Hf - Hi
Where,
- ΔH: enthalpy variation
- Hf: final enthalpy or product enthalpy
- Hi: initial enthalpy or reagent enthalpy
From this, we conclude that the enthalpy variation is negative when we are faced with an exothermic reaction. In turn, the enthalpy variation is positive when we are faced with an endothermic reaction.
Be sure to check these texts to learn even more about the topic.:
- Thermodynamics
- Calorimetry
- sensible heat
- latent heat
Exercises with commented feedback
1. (Udesc/2011) Given the following equations:
| (THE) | 2CO(g) + O2(g) → 2CO2(g) | ΔH = - 565.6 kj |
| (B) | 2CH4O(g) + 3O2(g) → 2CO2(g) + 4H2O(1) | ΔH = - 1462.6 kj |
| (Ç) | 3O2(g) → 2O3(g) | ΔH = + 426.9 kj |
| (D) | Faith2O3(g) + 3C(s) → 2Fe(s) + 3CO(g) | ΔH = +490.8 kj |
Consider the following propositions in relation to the equations:
I. Reactions (A) and (B) are endothermic.
II. Reactions (A) and (B) are exothermic.
III. Reactions (C) and (D) are exothermic.
IV. Reactions (C) and (D) are endothermic.
V. The reaction with the greatest release of energy is (B).
SAW. The reaction with the greatest energy release is (D).
Check the correct alternative.
a) Only statements II, III and V are true.
b) Only statements I, III and VI are true.
c) Only statements I, IV and VI are true.
d) Only statements II, V and VI are true.
e) Only statements II, IV and V are true.
Correct alternative: e) Only statements II, IV and V are true.
a) WRONG. Statement III is not true.
Contrary to statement III, reactions (C) and (D) are endothermic, as the positive sign in the enthalpy variation indicates heat absorption.
b) WRONG. None of the statements cited in this alternative are correct. They are wrong because:
- Reactions (A) and (B) are exothermic, as the negative sign in the enthalpy change indicates the release of heat.
- Reactions (C) and (D) are endothermic, as the positive sign in the enthalpy change indicates heat absorption.
- Reaction (D) does not release energy as it is endothermic.
c) WRONG. Of the three statements cited in this alternative, only IV is correct. The other two are wrong because:
- Reactions (A) and (B) are exothermic, as the negative sign in the enthalpy change indicates the release of heat.
- Reaction (D) does not release energy, the positive sign in the enthalpy change indicates that the reaction is endothermic.
d) WRONG. Statement VI is not true.
Contrary to statement VI, reaction (D) does not release energy, as it is endothermic.
a) CORRECT. The statements are correct because:
- Reactions (A) and (B) are exothermic, as the energy change is negative.
- Reactions (C) and (D) are endothermic, as the value of ΔH is positive.
- The reaction with the greatest release of energy is (B), since among the exothermic reactions of the utterance, this is the one with the highest value with a negative sign.
These texts will help you increase your knowledge:
- Exercises on Thermochemistry
- chemical transformations
- Chemical reactions
2. (Enem/2011) An unusual option for cooking beans is the use of a thermos. In a pan, place a part of beans and three parts of water and let the set boil for about 5 minutes, then transfer all the material to a thermos. Approximately 8 hours later, the beans will be cooked.
The cooking of the beans takes place inside the thermos because
a) water reacts with beans, and this reaction is exothermic.
b) beans continue to absorb heat from the surrounding water, as it is an endothermic process.
c) the considered system is practically isolated, not allowing the beans to gain or lose energy.
d) the thermos provides enough energy to cook the beans once the reaction starts.
e) the energy involved in the reaction heats the water, which maintains a constant temperature, as it is an exothermic process.
Correct alternative: b) beans continue to absorb heat from the surrounding water, as it is an endothermic process.
a) WRONG. A chemical reaction is characterized by the formation of new substances, which do not occur when cooking beans.
b) CORRECT. When water is heated, it gains heat and a thermos does not allow this energy to be lost to the environment. Thus, beans absorb heat from water and cook, featuring an endothermic process.
c) WRONG. The system is isolated from the external environment. Inside the bottle, the beans and water are having direct contact and, therefore, performing a thermal exchange.
d) WRONG. The thermos flask has the function of insulating the system, not allowing the mixture inside it to exchange heat with the environment.
e) WRONG. The temperature is not constant, as as water transfers heat to the beans, it loses energy until the two temperatures are equal.
Check out the texts below and learn more about the topics covered in this issue:
- Physical and chemical transformations
- heat and temperature
- Thermal energy



