Battery it is a system in which chemical energy is spontaneously transformed into electrical energy.
In 1836, the English chemist and meteorologist John Frederic Daniell (1790-1845) built a pile different from the one known at the time: the pile of Alessandro Volta. In this pile he interconnected two electrodes, which were systems consisting of a metal immersed in an aqueous solution of a salt formed by this metal's cations.
One of the electrodes, the copper electrode, consisted of a copper plate dipped in a solution of copper sulfate (CuSO4). The other electrode was a zinc electrode, consisting of a zinc plate immersed in a zinc sulfate (ZnSO4) solution.
These two electrodes were interconnected by an electrical circuit that contained a lamp, because if it turned on, it would indicate the emergence of an electrical current.
Also, there was a salt bridge between them. This bridge consisted of a U-shaped glass tube containing a concentrated aqueous solution of a highly soluble salt, such as potassium chloride (KCl (aq)), for example. The ends of the tube are coated with cotton or agar.
Below we have the structure of this cell or electrochemical cell, which became known as the Daniell cell. Remembering that each electrode is called a half cell.
With the circuit closed, the lamp turns on and after some time, the zinc plate is corroded and has its mass decreased, whereas the copper plate is the opposite, its mass increases (as shown in the figure below). It is also noted that there is an increase in the concentration in mol/L of the Zn ions2+ and a decrease in Cu ions2+.
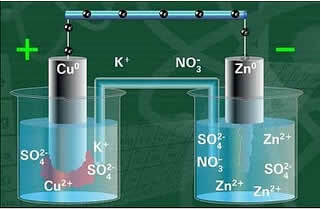
Daniell's Stack Schematic.
Why is this happening?
Do not stop now... There's more after the advertising ;)
To understand, let's look at the reactions that take place at each electrode separately.
In the Zinc electrode, the following equilibrium reaction occurs:
Zn(s) ↔ Zn2+(here) + 2 and-
This means that metallic zinc (from the plate) undergoes oxidation, that is, it donates two electrons to the zinc cation (from the solution) and becomes Zn2+. The opposite also occurs, the zinc cation present in the solution receives the two electrons donated by the zinc and becomes metallic zinc. Therefore, there is an uninterrupted process of oxidation and reduction.
The same applies to the copper electrode, which will have the global reaction in dynamic equilibrium: Cu(s) ↔ Cu2+(here) + 2 and-
Daniell realized that if he connected these two electrodes, the more reactive one would donate its electrons to the less reactive cation instead of doing this with the cations in its own solution. In this case, zinc is the most reactive and undergoes oxidation, donating electrons to copper, hence the decrease in its mass. The electrode that undergoes oxidation is the negative pole, called the anode. The copper electrode is the one that undergoes reduction, the copper cation receives the two electrons from zinc, and is called the cathode (positive pole).
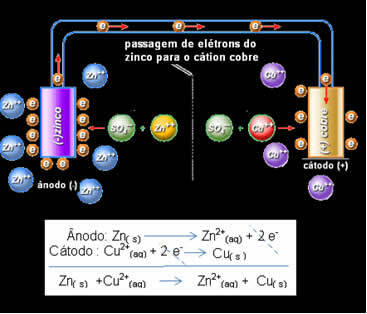
Electron passage and Daniell's stack global equation.
Above we have the global equation for this stack, which is obtained by adding the two half-reactions. Its representation or chemical notation is made according to the following rule:

So for Daniell's stack we have:
Zn / Zn2+// Ass2+ / ass
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Physicochemical - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Danill's Pile"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/pilha-daniell.htm. Accessed on June 28, 2021.