If we use a voltmeter in a battery, we will be able to identify the difference in potential (U or ddp) or electromotive force (emf or E) between the two electrodes. However, it is not possible to identify the reduction or oxidation potentials of each electrode in this way.
Scientists needed to know these values to study oxidation-reduction processes, so they established a reference state. This means that it was agreed to measure the potential of each electrode in relation to another electrode under the following standard conditions:
• The temperature must be at 25°C;
• Pressure at 1.0 atm;
• The concentration of the solution in which the metal is immersed must be 1.0 mol/L.
Thus, the chosen electrode was the hydrogen electrode, which is represented below:
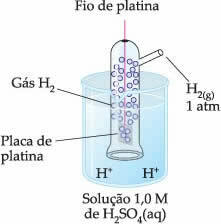
This electrode is composed of a platinum wire connected to a platinum plate, which does not participate in the reaction, inside a tube containing hydrogen gas and immersed in an acidic solution. In the example, the solution was sulfuric acid.
| By convention, the standard hydrogen electrode has been assigned the value zero, so much for the E0red as for the E0oxy. |
Thus, to find the potential value of any other electrode we just build a stack of the electrode we want with the standard hydrogen electrode and measure the ddp with a voltmeter. The value displayed on the voltmeter will be the potential of the sought electrode, since that of hydrogen is equal to zero.
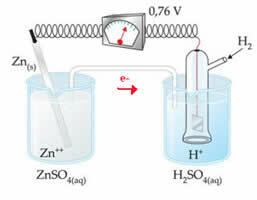
For example, we interconnect a zinc electrode with the hydrogen electrode to find out what its reduction potential is:
Do not stop now... There's more after the advertising ;)
According to the above scheme, the voltmeter identified the potential difference as being equal to +0.76(?E0 = +0.76). We also note that the zinc electrode has oxidized, so it is the anode; and the hydrogen electrode reduced, being the cathode.
So we have:
?E0 = E0red (cathode) - E0 red (anode)
0.76 = 0.00 - E0 red (Zn)
E0 red (Zn) = 0.00-0.76
E0 red (Zn) = -0.76
The negative value means that the electron current flows from the zinc electrode (anode) to the hydrogen electrode, thus behaving like a cathode. If it were positive, it would be the other way around, and the hydrogen electrode would behave like an anode. This can be seen when we interconnect a copper electrode with the standard hydrogen electrode:
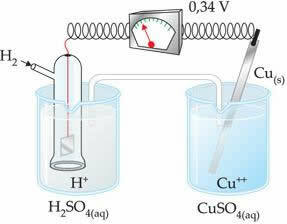
?E0 = E0red (cathode) - E0 red (anode)
-0.34 = 0.00 - E0 red (Zn)
E0 red (Zn) = 0.00+0.34
E0 red (Zn) = +0.34
Thus, it is possible to define the reduction and oxidation potentials for the most varied chemical species. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends the use of reduction potentials only. And, regardless of the metal used, in the battery representation, the hydrogen electrode always comes first, for example:
Pt – H2 (g) 1atm / H3O1+ (aq) 1 mol/L // Cu2+ (aq) 1 mol/L / Cu
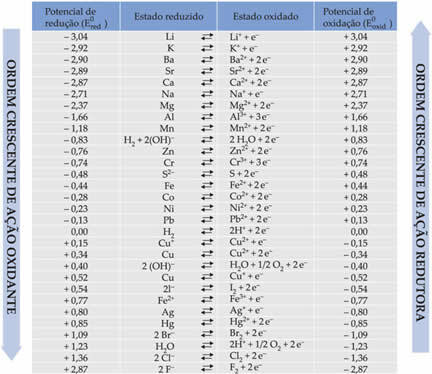
Listed below in the table are the potentials achieved through this method of using the standard hydrogen electrode, together with their respective semi-reactions:
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Measurement of electrochemical potentials"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/medicao-dos-potenciais-eletroquimicos.htm. Accessed on June 28, 2021.

