Aluminum is obtained through metallurgical processes. Metallurgy is an area that studies the transformation of ores into metals or metallic alloys. Several metals are obtained by this method, such as copper, titanium, iron and manganese.
In the case of aluminum, the main ore used is the bauxite (figure), which contains hydrated aluminum oxide (Aℓ2O3. x H2O) and various impurities.

In aluminum metallurgy, the following four steps occur:
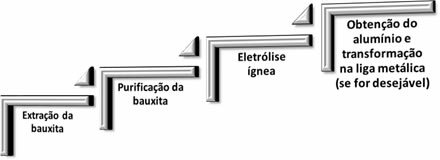
When aluminum oxide (Aℓ2O3(s)) is separated from bauxite, its name becomes alumina.
Previously, the following was done: alumina was treated with hydrochloric acid to generate aluminum chloride; which was placed to react with metallic potassium or sodium, causing the reduction of the compound and giving rise to metallic aluminum:
Aℓ2O3(s) + 6 HCℓ(here)→ 4 AℓCℓ3(aq) + 3 H2O(ℓ)
ACℓ3(aq) + 3K(s)→ 3 KCℓ(s) + Aℓ(s)
However, this method was very expensive and inefficient, so aluminum was considered a rare metal.
But in 1886, two scientists separately developed the method mentioned above, in which igneous electrolysis was used. These scientists were the American Charles M. Hall and the Frenchman Paul Héroult, so this method came to be called
Hall-Héroult Process or simply,Hall process, whereas Charles M. Hall patented it.The key point they discovered was how to make aluminum oxide liquid to do so. be able to perform its igneous electrolysis, as the problem was that its melting point was above 2000°C. They used a flux, cryolite ore (Na3AℓF6), which was able to lower the melting temperature of aluminum oxide to about 1000 °C.
Thus, as shown in the diagram below, this mixture of aluminum oxide and cryolite was placed in a carbon-lined steel electrolytic vessel. An electric current passes through this molten mixture. The walls of the container that are in contact with the mixture act as a negative pole of electrolysis (cathode), where the reduction of aluminum cations occurs. The anode (positive pole) are cylinders made of graphite or carbon, that is, both made of carbon, where the oxidation of oxygen anions occurs:
Do not stop now... There's more after the advertising ;)
Cathode half-reaction: 4 Aℓ3+(ℓ) + 12 and- → 4 Aℓ(ℓ)
Anode half-reaction: 6 O2-(ℓ) → 12 and- + 3 O2(g)
The oxygen formed reacts with carbon in the anode and also generates carbon dioxide:
3 O2(g) + 3 C(s) → 3 CO2(g)
So the overall reaction and scheme of this igneous electrolysis that gives rise to aluminum is given by:

The aluminum obtained is in liquid form, because its melting point is 660.37 ºC, that is, lower than that of a mixture of alumina and cryolite. Aluminum is also denser than the mixture and, therefore, it is deposited at the bottom of the container, where it is collected.
In the production of 1 ton of aluminum it is used:
- 4 to 5 tons of bauxite, from where about 2 tons of alumina;
- 50 kilograms of cryolite (there are not many natural reserves of cryolite, therefore, it is usually obtained through its synthesis from fluorite (CaF2), a most abundant mineral in nature);
- 0.6 tons of coal for the electrodes.
Annually aluminum production exceeds 27.4 million tons.
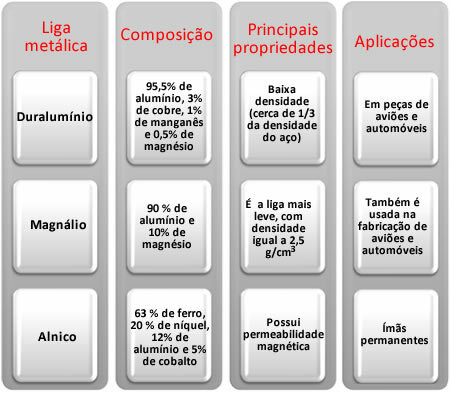
Among the main aluminum alloys, we have the following:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Obtaining aluminum through electrolysis"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/obtencao-aluminio-por-meio-eletrolise.htm. Accessed on June 28, 2021.
Chemistry

Applications of Electrolysis, electroplating, nickel plating, chrome plating, nickel, chromium, cathode, sodium, aluminum, chlorine, caustic soda, hydrogen gas, igneous electrolysis, aqueous electrolysis, alkali metals, alkaline earth, gas chlorine.