THE salt bridge was proposed by the English chemist Frederic Daniell, in 1836, when this scholar set up the battery named after him (Danill's Pile). this bridge is a U-shaped tube that has two porous ends. (consisting of cotton or agar-agar) and contains an aqueous solution formed by water and a salt. It indirectly connects the solutions of the two half cells of the cell (the anode and the cathode).
Observation: The anode is the negative pole of the battery and is where oxidation occurs, and the cathode is the positive pole and where reduction occurs.
To understand the importance of the salt bridge, it is initially necessary to understand the functioning of the pile. See the diagram below:
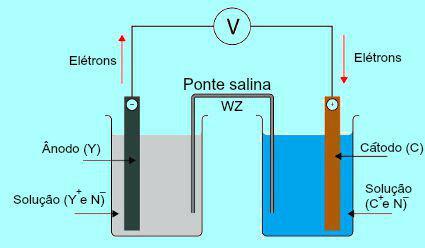
General scheme of a Daniell stack
Initially, the metal (Y) of the plate present in the anode undergoes oxidation. When undergoing oxidation, the metal turns into cation, which falls into the solution in which the plate is. For this reason, the anode solution starts to present a higher concentration of cations (Y+). In this case, a decrease in the anode metal plate is observed.
Y → and- + Y+
Then, the electrons lost during the oxidation of the metal of the anode plate travel through the external electrical wire and go to the metal plate present in the cathode. Thus, the plate present at the cathode becomes charged with electrons.
-
The present cations (C+) in the solution move towards the cathode plate because it is charged with electrons. The cations, when gaining the electrons present in the plate, become neutral (stable and solid) and adhere to it. For this reason, the cathode solution presents a higher concentration of anions than of cations. In this case, an increase in size of the cathode metal plate is observed.
Do not stop now... There's more after the advertising ;)
Ç+ + and- → C
From the understanding of how the Daniell's pile, it is possible to observe that the solution present in the anode receives a greater amount of Y cations+ due to the oxidation of the metal of the X plate. The solution present in the cathode, on the other hand, loses its cations (C+) because they suffer from reduction in plaque.
It is precisely on this issue that the work of the salt bridge is highlighted. The main function of this bridge is to promote the balance of charges present in both the anode and cathode solutions. In the salt bridge, we always have a salt (usually potassium chloride or ammonium nitrate.) dissociated in water. See the representation of an equation in the salt bridge:
WZ + H2O → W+ + Z-
In the salt bridge, there are two ions (W+ and Z-), which are shifted to the cathode and the anode according to:
The) The anode receives the anions (Z-) present in the salt bridge due to the oxidation of the element (Y), which causes an increase in the amount of cations (Y+) in the solution.
B) The cathode receives cations (W+) of the salt bridge because, during battery operation, the cation (C+) present in the solution is reduced on the board. Thus, the cathode solution has a greater amount of anions (N-) and therefore receives the cation present in the salt bridge.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Sail bridge"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/ponte-salina.htm. Accessed on June 28, 2021.

