Halogen lamps (also called halogen lamps) are widely used by professionals in the architecture and interior design to create different environments that require more lighting points intense.
These lamps have the same operating principle as incandescent lamps, that is, they are generally formed by an ampoule with a thin filament of tungsten (W), metal with a very high melting point, withstanding high temperatures. When this tungsten filament is traversed by electrical current, it emits a white light with a slightly yellowish tinge.
Over time, the tungsten sublimes (changes from a solid to a gaseous state) from the filament and deposits in the bulb, darkening the bulb:
W(s) ↔ W(g)
However, the difference between common incandescent lamps and halogen lamps is that the latter contain gaseous iodine inside. Iodine is from the family of halogens in the periodic table, hence the name given to this type of lamp.
In the filament, the iodine reacts with the gaseous tungsten that is released, forming the tungsten iodide gas:
Do not stop now... There's more after the advertising ;)
W(g) + 3 I2(g) ↔ WI6 (g)
When this gas approaches the bulb, which is a cooler region, it decomposes, thus recovering the metallic tungsten (solid), which is again deposited on the filament.
WI6 (g) ↔ W(s) + 3 I2(g)
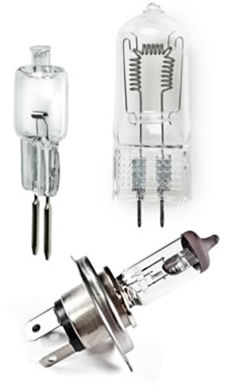
The result is that this type of lamp does not dim and lasts longer. Ordinary incandescent lamps last an average of 1 year or 1000 hours, while halogen lamps last 2000 hours and can reach 5000 hours. They are also a little more efficient or economical than incandescent ones. But, it is noteworthy that they consume more energy compared to fluorescent or discharge.
These points and the fact that its light is brighter than the common incandescent is what makes its use to be growing more and more.
One type of halogen lamp is the dichroic lamp, which has a reflector that reduces the excessive heat produced.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Chemical balance in halogen lamps"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/equilibrio-quimico-lampadas-halogenas.htm. Accessed on June 28, 2021.
Chemistry

Test your knowledge and learn more with this list of solved exercises on chemical balances. Through this material, you will be able to better understand how to work equilibrium constants (Kp, Kc and Ki), equilibrium shift, pH and pOH, as well as equilibrium in so-called buffer solutions.