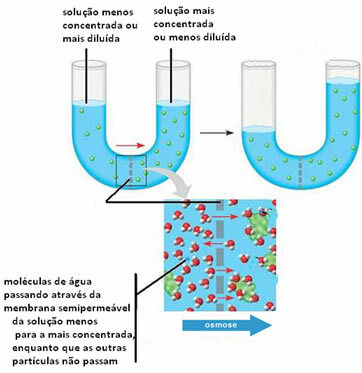
Osmosis is a colligative property that works as follows:
The passage of solvent to a solution or the passage of solvent from a diluted solution to a more concentrated one occurs, through a semipermeable membrane.
Below we can see a fantasy color representation of the passage of pure solvent into a solution.

In the following case, we have the passage of the solvent (water molecules) from a diluted solution to a more concentrated one:
This is what happens, for example, when we put a carrot in a jar with a concentrated solution of water and salt. After approximately two days, it is noted that the carrot has less volume and a wrinkled appearance and shriveled up, because the water molecules in their cells are transferred to the brine, which is more concentrated. Water molecules pass through carrot cell membranes.
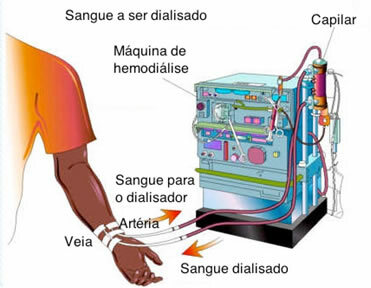
The idea of this process is used in medicine, in hemodialysis. Although, the difference lies in the fact that in ordinary osmosis, through semipermeable membranes, only the solvent passes and the solute is retained. In hemodialysis, however, both the solvent (water) and the solute particles (among them, the toxic waste produced in our body) pass through the semipermeable membrane used.
Do not stop now... There's more after the advertising ;)
The filtering membrane of the artificial kidney is formed by capillary filters, which are a set of thin tubes.
Dialysis is a slow process that has great therapeutic application, especially for patients with renal impairment, whether acute or chronic. Through the kidneys we eliminate toxic waste produced in the body, such as urea, nitrogen compounds and creatine. Those who have this problem, however, need to undergo hemodialysis sessions from 4 to 7 hours a day. And it can be for a person's lifetime if he doesn't get a transplant.
The process is as follows: the person's blood is pumped into a tube, which is lined with a semipermeable membrane (dialyzer membrane). This tube is immersed in a solution with blood plasma components. Thus, osmosis occurs: the particles of toxic waste present in the person's blood pass through the membrane, being eliminated. And the clean blood goes back to the person's body.
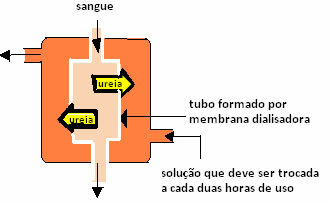
Blood cells, proteins, and other blood components do not participate in osmosis because their particle size is larger than the membrane's pores.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Osmosis in Medicine – Hemodialysis"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/osmose-na-medicina-hemodialise.htm. Accessed on June 28, 2021.