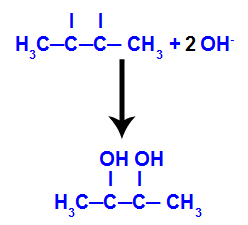
Alkanes are hydrocarbons that have only single bonds and open chains, that is, they are saturated and acyclic.
These compounds are also called paraffinic hydrocarbons or paraffins.
The general formula for alkanes is CnoH2n+2.
Alkanes are responsible for forming oil and natural gas. They are also important fuels like cooking gas and gasoline.
Features
The main characteristics of alkanes are:
- colorless
- Not very reactive, as the link between C and H is quite stable
- oil odor
- Insoluble in water
- Soluble in organic solvents such as ether, alcohol and benzene
- Melting, boiling and density points increase with molecular weight
Learn more, read also:
- alkenes
- Alkynes
- Cyclans
- Alkadienes
Nomenclature
The nomenclature of alkanes is given as follows:
PREFIX + INFIX + SUFFIX
The prefix indicates the amount of carbons in the main chain.
The infix is given by the term "an", which represents single links. The suffix is given by the letter "o", which indicates the hydrocarbon compound.
In summary, to demonstrate that the compound is an alkane, the ending "year".
Unbranched alkanes
When the alkane chain is not branched, it is terminated YEAR.
Examples
| Name | molecular formula |
structural formula |
|---|---|---|
| Methane | CH4 | CH4 |
| Ethane | Ç2H6 | CH3 — CH3 |
| Propane | Ç3H8 | CH3 — CH2 — CH3 |
| Butane | Ç4H10 | CH3 — (CH2) — CH3 |
| pentane | Ç5H12 | CH3 — (CH2)3 — CH3 |
| Hexane | Ç6H12 | CH3 — (CH2)4 — CH3 |
| Heptane | Ç7H16 | CH3 — (CH2)5 — CH3 |
| Octane | Ç8H18 | CH3 — (CH2)6 — CH3 |
| ninth | Ç9H20 | CH3 — (CH2)7 — CH3 |
| dean | Ç10H22 | CH3 — (CH2)8 — CH3 |
know more about:
- Nomenclature of hydrocarbons
- Organic compounds
- Organic chemistry
branched alkanes
When dealing with branched alkanes, the branches must also be indicated.
The branches of alkanes can be simple as a result of the removal of a hydrogen atom.
The branch name is derived from the corresponding alkane, changing the suffix "year" to "il" or "ila". Hence, they are called alkyl radicals.
Examples:
Methane (CH4): If a hydrogen atom is removed, it becomes methyl (CH3).
Ethane (CH3 – CH2):With one less hydrogen atom it turns into ethyl (CH2 — CH3).
Remember that the main chain is the one with the highest number of carbons. Also, the branches should be numbered so that they are given as few as possible.
2-methylheptane
Keep studying! Learn more about Hydrocarbons:
- Carbon
- Aromatic Hydrocarbons
- Benzene
- Butane
- Petroleum
- Exercises on Hydrocarbons