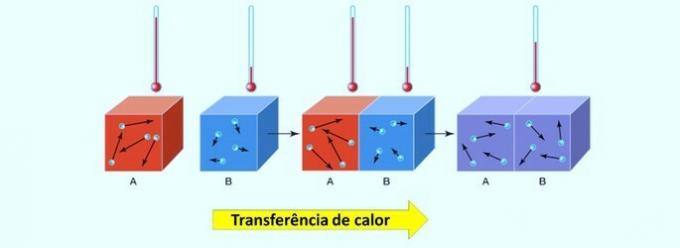
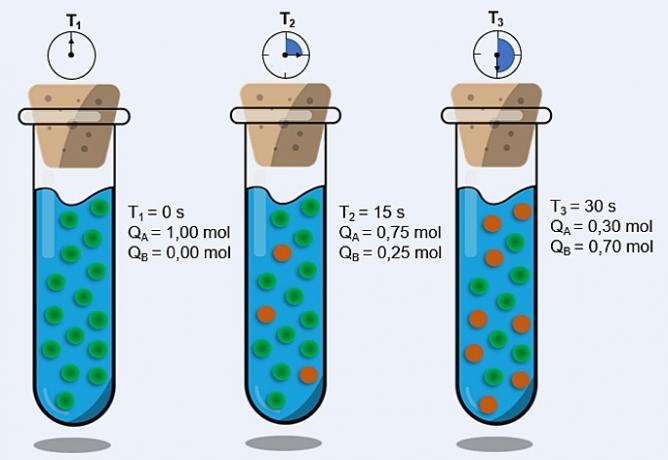
The higher the temperature, the greater the speed of a reaction.
This can be easily seen in many situations in our daily lives, as in the following examples:
- When we want to slow down a food's decomposition reaction, we lower the temperature, placing it in the refrigerator;

- If we want to speed up the cooking reaction of the food, just put it in a cooking pot. pressure which, with increasing pressure, also increases the boiling temperature of the liquid water in which the food is;

- Fires, in general, are usually devastating because the temperature of the environment increases, which causes an increase in the speed of the combustion reaction;
- To slow down metabolic chemical processes, decreasing the chances of brain damage due to oxygen deficiency, some surgeries are performed by lowering the patient's body temperature, remaining at around 15°C;
- If we put an effervescent tablet in a glass of hot water and another in a glass of cold water, the first one will dissolve much faster.
But what explains the directly proportional influence of temperature on the reaction rate?
Do not stop now... There's more after the advertising ;)
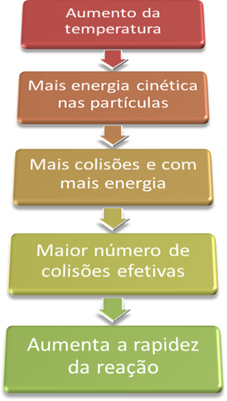
This is because, as explained in the text “Conditions for the Occurrence of Chemical Reactions”, for a reaction to proceed, it is necessary to satisfy some conditions, such as that the particles they must collide effectively and with the minimum energy required, which is called activation energy.
Thus, when we increase the temperature of the system, we also increase the agitation of the reacting particles and provide them with more kinetic energy. With this, more collisions will occur and with more energy, increasing the amount of particles that will react and, consequently, increasing the reaction speed.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Temperature and Speed of Reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/temperatura-velocidade-das-reacoes.htm. Accessed on June 27, 2021.