THE origin of Periodic table occurred in the early 19th century, around 1829, when chemists at the time decided to propose ways of organizing the chemical elements known until then.
At the beginning of the 19th century, chemists had knowledge about several characteristics (density, atomic mass, reactivity, melting point, boiling point, physical state) of thirty chemical elements. This knowledge served as a starting point for the origin of the Periodic Table.
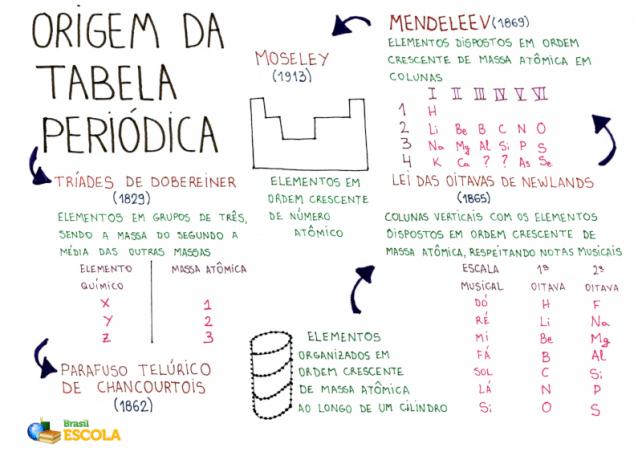
Over 200 years, several chemists sought to propose ways to organize the chemical elements, that is, the The Periodic Table we know today actually had various origins, as throughout history many attempts were performed.
Mind Map: Origin of the Periodic Table
*To download this mind map, Click here!
Here are some of the chemists who have excelled in trying to organize the elements into a table.
Dobereiner Triads

Illustration by Johann Wolfgang Dobereiner *
In the year 1829, the German chemist Johann Wolfgang Dobereiner organized the first Periodic Table in history. It featured the thirty chemical elements known until then and was named by him the Dobereiner triads.
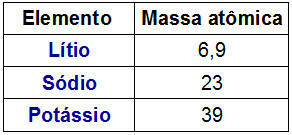
THE Dobereiner periodic table it was called a triad because the elements were organized in groups of 3. Each group had elements that had similar chemical characteristics.
Representation of a Dobereiner triad
An interesting fact about Dobereiner triads is that the atomic mass of the central element of the triad was exactly the result of the arithmetic mean between the atomic masses of the other two elements of the triad.

Telluric screw by Alexandre de Chancourtois
In the year 1862, the French geologist and mineralogist Alexandre de Chancourtois he decided to propose an organization of the chemical elements known at the time to facilitate their application in mineralogy. THE Chancourtois table it was called telluric screw.

Representation of the Chancourtois telluric screw
Chancourtois distributed the chemical elements (dark spots in the image) in ascending order of atomic mass along a spiral band existing in a cylinder. With this organization, Chancourtois observed that elements positioned on the same vertical line had similar chemical properties.
Law of Octaves
Do not stop now... There's more after the advertising ;)
Law of octaves was the name proposed by the English chemist J.A.R. Newlands, in the year 1865, to the Periodic Table. Because Newlands is also a musician, he set up the table according to the musical notes (do, there, re, mi, fa, sol, there, si).
Newlands organized the 61 chemical elements known at the time in ascending order of atomic mass and placed them in vertical columns. Each of the vertical columns had seven elements.
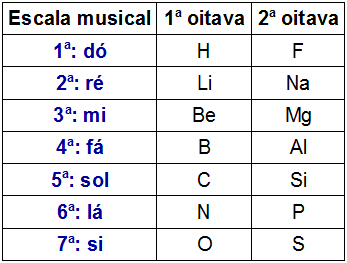
Two octave representation of Newlands
Newlands observed that chemical elements present in the same horizontal line of different octaves had similar chemical properties. Thus, the first element of an octave had similar properties to the first element of the other octave, and so on.
Mendeleev's Periodic Table

Illustration by the chemist Mendeleev **
Mendeleev, during his work with the chemical elements, he was in the habit of writing down the properties of each one of them on cards. At some point, in the year 1869, he decided to place these tokens in ascending order of atomic mass.
Right after arranging the elements in ascending order of atomic mass, Mendeleev kept the pattern, but he positioned the elements in horizontal and vertical columns, respecting the characteristics and similarities of the elements.
Moseley Periodic Table
In the year 1913, the English chemist HenryMoseley, based on the table proposed by Mendeleev, he assembled the periodic table in the patterns we know today.
Unlike Mendeleev, Moseley arranged the elements in ascending order of atomic number, kept the organization in horizontal and vertical columns, but placed the elements with the same chemical characteristics in the same vertical columns.
current periodic table
After 1913, the Periodic Table proposed by Moseley did not undergo any major changes, in fact, it underwent some updates, as some chemical elements were discovered.
Comparing it with the current table, the Moseley table did not show, for example, the chemical elements with atomic numbers between 110 and 118. Furthermore, the actinide series was located above the lanthanide series.
THE last update held in the Periodic Table was in 2016, when elements 113, 115, 117 and 118 officially became part of it.
* Image credit: Yangchao/ shutterstock. Inc
** Image credit: Olga Popova/ shutterstock. Inc
By Me. Diogo Lopes Dias