Surface tension is a phenomenon that occurs on the surface of liquids, such as water, forming a thin film.
When water, in a liquid state, occupies a container, we can perceive the separation between the liquid and the environment. This is because the interaction between water molecules on the surface is different from the interactions inside the liquid.
On the surface, a water molecule interacts with molecules on the sides and below it. Inside, a molecule is surrounded by other molecules and there is interaction in all directions through hydrogen bonds.
It is because of this property that we observe the phenomenon of the formation of a droplet. Because of this, it is also possible for insects to walk on water.
What is surface tension?
It is the formation of a thin film under a liquid due to the inequality of attractions between the molecules that make it up. This phenomenon occurs more markedly in liquids that have intermolecular forces intense, like water.
Interactions between species in a liquid are called cohesive forces. Whereas molecules inside a liquid are attracted to neighboring molecules in all directions, molecules on the surface interact with molecules below and beside them.
See how surface tension occurs in water.
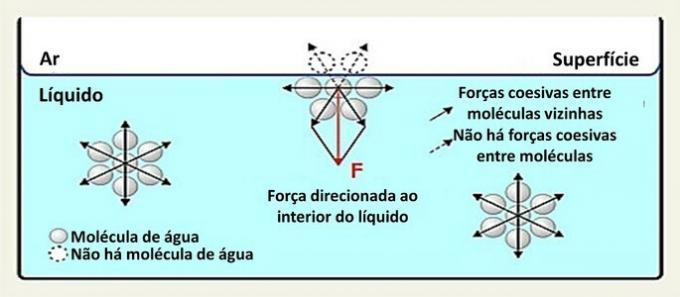
The water (H2O) is a polar molecule formed by 2 hydrogen atoms (positive poles) and one oxygen atom (negative pole) joined by covalent bonds. The positive pole of a molecule is attracted to the negative pole of the neighboring molecule, forming hydrogen bonds.
This type of interaction within the liquid is distributed in all directions. On the surface, the forces are directed downwards and sideways, because above them there are no water molecules. This makes the surface molecules more cohesive and creates an elastic film.
The surface tension unit is given by the quotient between unit of force and unit of length, with the most adopted being dyne/centimeter (dyne/cm) and newton/meter (N/m).
Water has a high surface tension, the value of which is 72.75 dyne/cm. However, mercury, a liquid metal, has a surface tension approximately 7 times greater than water, 475 dyne/cm.
Want to know more? So check out the following texts:
- Water Properties
- Polar and Apolar Molecules
- Chemical bonds
Phenomena caused by surface tension
Surface tension is responsible for some phenomena we observe in everyday life. The main ones are:
Animals that walk on water

Insects, spiders and other animals can walk or rest on the Water because on the extremities of its paws there are hairs coated with a greasy substance and, therefore, they cannot penetrate between the water molecules that are joined on the surface.
Formation of water droplets

The water droplets are spherical due to the contraction in the molecules of the surface caused by surface tension. The sphere occurs because this is the geometric shape in which there is the smallest relation between surface area and volume. Therefore, the spherical shape maintains the fewest number of water molecules in contact with the air.
Exercises on surface tension of water
1. A surfactant is a substance that acts on another to change:
a) Osmolarity.
b) Surface tension.
c) Electrophoresis.
d) Viscosity.
e) Osmotic pressure.
Correct alternative: b) Surface tension.
a) WRONG. Osmolarity is related to the amount of solute particles that are contained in a given volume of solvent.
b) CORRECT. Both detergents and soaps lower the surface tension of water and are generically called surfactants, because the molecules of these materials are placed between the water molecules and reduce the tension superficial.
c) WRONG. Electrophoresis is a technique for separating molecules according to their charges.
d) WRONG. Viscosity is a physical property that determines a fluid's resistance to flow.
e) WRONG. Osmotic pressure is a colligative property that corresponds to the pressure that must be exerted on a system to prevent osmosis from occurring spontaneously.
Learn more about the issues covered in this issue:
- Matter Properties
- Colligative Properties
- Osmotic Pressure
2. The surface tension of liquids depends directly on interaction processes between molecules, such as hydrogen bonding, for example. Which of the substances below has the highest surface tension?
a) benzene
b) octane
c) ethyl alcohol
d) carbon tetrachloride
e) ethanoic acid
Correct alternative: e) ethanoic acid.
a) WRONG. Benzene is a hydrocarbon, a non-polar molecule, and does not make hydrogen bonds.
b) WRONG. Octane is a hydrocarbon and, therefore, it is a non-polar molecule that does not make hydrogen bonds.
c) WRONG. Ethyl alcohol is a slightly polar compound that can form hydrogen bonds, but the interaction between the molecules is limited.
d) WRONG. Carbon tetrachloride is a non-polar organic compound and therefore does not make hydrogen bonds.
e) CORRECT. The carboxylic acid functional group (-COOH) can make hydrogen bonds with either the oxygen or the hydroxyl hydrogen.

Learn more about the issues covered in this issue:
- Polarity of Molecules
- Organic Functions
- Hydrocarbons
surface tension experiment
Watch the video below with an experiment that demonstrates the surface tension of water.


