It has long been known that when sunlight passes through a prism, similar to the one in the figure above, scattering of the light components occurs. This set of colors ranging from red to violet is known as continuous spectrum, as the transition from one color to another is practically imperceptible.
These colors make up what we call the visible light or visible radiation, which are composed of electromagnetic waves. I.e, waves formed by oscillations in the electric field and the magnetic field that occur simultaneously, being perpendicular to each other.
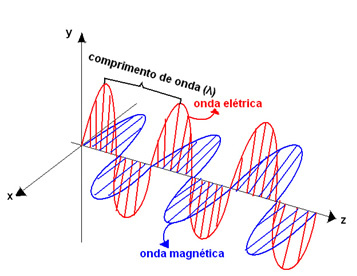
These waves have frequencies (f) – number of vibrations of this wave per second – and wave-length – the distance from the crest of one wave to the other, represented by the Greek letter lambda (λ). Thus, the difference between one color and another is the frequency and wavelength of each electromagnetic wave that makes up the colors.
However, this phenomenon of observing the spectrum is not only obtained with sunlight. We can also make other lights pass through a prism. So we'll get other spectra. However, these
spectra will be discontinuous, with spacing between colors, which we call in the spectrum as streaks or bands.Do not stop now... There's more after the advertising ;)
Let's say, for example, that we let the light emitted from a gas discharge tube, filled with hydrogen gas, pass through a prism. The spectrum obtained would be similar to the one shown below.

If it were the gas of another element, the spectrum would also be discontinuous, but it would look different. In this way, each spectrum serves as a “digital” for the identification of chemical elements; for each has a different spectrum; never repeats.
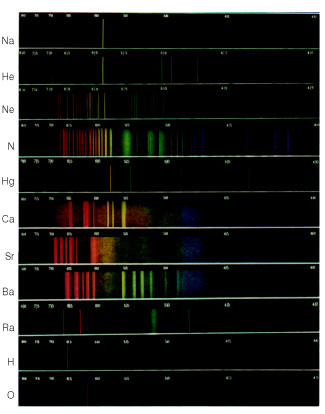
Nowadays it is possible to obtain and visualize the spectra of the elements through a device called spectroscope.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Electromagnetic Spectrum of Chemical Elements"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/espectro-eletromagnetico-dos-elementos-quimicos.htm. Accessed on June 27, 2021.