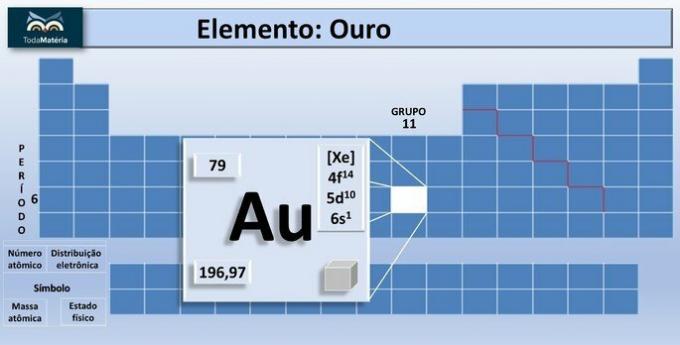
Gold is a chemical element of the Periodic Table represented by the symbol Au, whose atomic number is 79 and belongs to the transition metals.
It is one of the first metals that were manipulated by man, due to the fact that it is found pure in nature.
As a noble metal, gold is one of the most desired metals and is widely used in making jewelry, coins and ornamental objects in the form of an alloy with other metals.
Gold features
- It has a bright yellow color
- It is corrosion resistant
- Occurs freely in nature in the form of nuggets or grains
- Soft and flexible metal
- not very abundant in nature
gold properties
Gold has many applications due to its properties, which go beyond its luster and color. It is an easy metal to work and to be molded and, therefore, it has been used by man for a long time.
Physical properties
| Electric conductivity | 45.2 x 106 y/m |
|---|---|
| Density | 19.3 g/cm3 |
| Toughness | 2.5 (Mohs Scale) |
| Fusion point | 1064 °C |
| Boiling point | 2856 °C |
Chemical properties
| electronegativity | 2,54 |
|---|---|
| ionization energy | 9.226 eV |
| Oxidation numbers (Nox) | +1, +3 |
| Reactivity |
undergoes oxidation:
|
| most common compounds |
|
origin of gold
Due to its characteristics, the records of the exploitation of gold by man go back 6 thousand years. It is possible to see in the Bible the use of gold as a symbol of wealth and Egyptian hieroglyphics date the use of gold from 4000 BC. Ç.
This metal is linked to the culture and history of many peoples as it was discovered by various groups in different places and times.
In antiquity, there are records of gold exploration in Sudan, northern Greece, Iran and China.
In the Middle Ages, in addition to the discovery of this metal in other places, such as Austria and Saxony, the movement called the Alchemy, which sought to transform base metals into high-value materials, such as gold.
From the 11th century onwards, it is possible to perceive the expansion of this metal throughout the world, becoming widely used in the minting of coins.
Even in America, after its discovery, it was observed that the peoples inhabiting some regions, such as the Incas and Aztecs, possessed exploration reserves not only of this metal, but also of silver, which led to the rapid exploitation of the Spaniards in the continent.
In Brazil, in the regions of Minas Gerais, Mato Grosso and Goiás, gold mines were found, which resulted in a "gold rush" becoming an economic activity in the colonial period of the country.
What is gold for?

Jewelry
The biggest consumption of gold is for jewelry making. Color, luster, durability and the tradition of using this metal make a jewel that contains gold valuable.
To increase the strength of the material, artisans prepare an alloy with other metals such as platinum, silver and copper.
Carat was developed to specify the amount of gold in an alloy. For example: 24 karat gold (24K) is pure gold, while 12 karat gold (12K) is an alloy in which 50% of its composition belongs to this metal.
Coins
Gold has long had commercial value and has been used as a medium of exchange or money. This is due to its rarity, high value and possibility of being fractionated.
The first gold coins were made in 560 BC. Ç. by order of King Croesus of Lydia (a region of present-day Turkey).
There are also gold bars, which are still a form of investment for some institutions, due to their ease of handling and storage.
Electronics
Because it is corrosion resistant and has high conductivity, gold is used in electronics that use very low currents and voltages, giving the material reliability.
Sophisticated electronic devices such as cell phones, GPS (Global Positioning System) and calculators have a small amount of gold in their composition.
Learn more about the periodic table and other chemical elements at:
- Periodic table
- Chemical elements
- Families of the Periodic Table
- History of the Periodic Table


