You carboxylic acids are organic compounds characterized by presence of a carboxyl (COOH), are present in citrus fruits, vinegar, pharmaceuticals and preservatives.
You acids carboxylic participate in reactions, as esterification, used in the production of flavorings, among others. The characteristics of the compounds of this functional group vary. according to the size and structure of the carbon chain.
Read more: Acetylsalicylic acid - compound with carboxylic acid and ester groups

Characteristics of carboxylic acids
- Carboxylic acids are chemically represented by RCOOH or CO2H, where R is the organic radical attached to a carbonyl (C = O) and a hydroxyl (-OH).
- The presence of carboxyl (-COOH) confers a difference in polarity to the molecule, making it a polar compound.
- THE solubility and appearance vary with the number of carbons in the chain. Carboxylic acids with:
- up to four carbons are colorless and liquid, miscible in water;
- five to nine carbons are viscous, colorless liquids, sparingly soluble in water;
- 10 or more carbons are white solids, insoluble in water.
- Open-chain acids with up to six carbons have a strong odor and characteristic of rancid butter, they are relatively volatile.
- Carboxylic acid is the organic function with the most accentuated acid character, the compounds of this function have a pH between 3 and 5.
Classification of carboxylic acids
Carboxylic acids can be classified by the number of carboxyls present in the molecule:

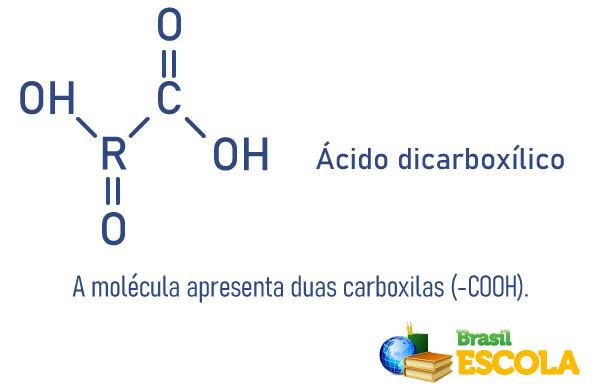
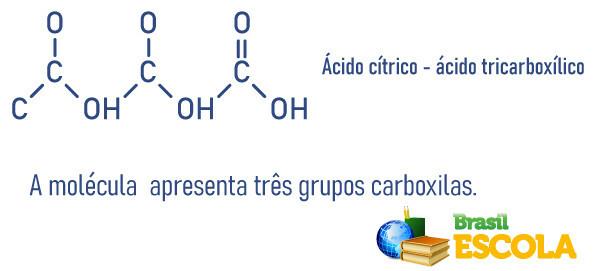
See too:Sulfuric acid - substance widely used by industries
Nomenclature of carboxylic acids
The nomenclature for carboxylic acids, according to the International Union of Pure and Applied Chemistry (Iupac), is characterized by the function start with the term acid and end with the suffix -oic.
The rules for nomenclature of this role, because it is a organic function, that is, by the presence of a carbon chain, they are:
1st step: number the carbons in the main chain, starting from the end where the carboxyl (-COOH) is located. The nomenclature prefix will be given according to the number of carbons in the main chain.
2nd step: name and locate branches: prefix (number of carbons) + termination (-il or -ila). If there is more than one branch in the chain, they must be placed in the naming alphabetically.
3rd step: check the existence of establishments in the chain.
Observation: The prefixes di-, tri- or penta- are used to express the presence of two, three or four groups of the same species. Example: dioic acid presence of two carboxyl groups.
See in the table below how the nomenclature for carboxylic acids should be structured:
Prefix (no. of carbons) |
Infix (chain saturation) |
Suffix (functional group) |
||||
Acid |
1 carbon |
Met- |
Single calls only |
-an- |
carboxylic acid |
-Hi co |
2 carbons |
Et- |
|||||
3 carbons |
Prop- |
1 double bond |
-en- |
|||
4 carbons |
But- |
|||||
5 carbons |
pent- |
2 double bonds |
-dien- |
|||
6 carbons |
Hex- |
|||||
7 carbons |
Hept- |
1 triple bond |
-in- |
|||
8 carbons |
Oct- |
|||||
9 carbons |
Non- |
2 triple links |
-diin- |
|||
10 carbons |
Dec- |
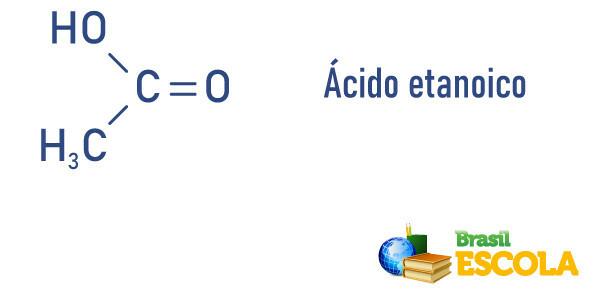
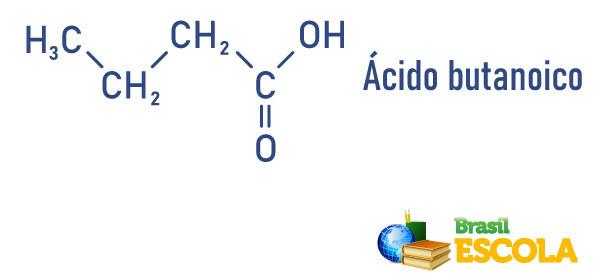
Examples:




Carboxylic acids present in everyday life
- Carbonic acid (OHCOOH): present in blood and carbonated beverages.
- Acetic Acid or ethanolic (CH3COOH): present in vinegar.
- Butyric or butanoic acid (CH3(CH2)2COOH): present in milk and dairy products.
- formic acid or methanolic (HCOOH): present in the venom of the red ant.
- Valeric or pentanoic acid (CH3(CH2)3COOH): drug used as a calming/sedative, also applied in the treatment of acne.
- Benzoic acid or carboxylic benzene (Ç6H5COOH): used by the food industry as a preservative and medicinally as a fungicide.
- Citric acid or 2-hydroxy-1,2,3-propane tricarboxylic acid: present in citrus fruits such as orange and lemon.
See too: Tips to determine-if the strength of an acid

Acid Reactionscarboxylic
dimers
Carboxylic acids tend to bind through the formation of hydrogen bonds, forming a dimer, in which two molecules start to behave like one only molecule, with double the molecular mass.
Neutralization reaction
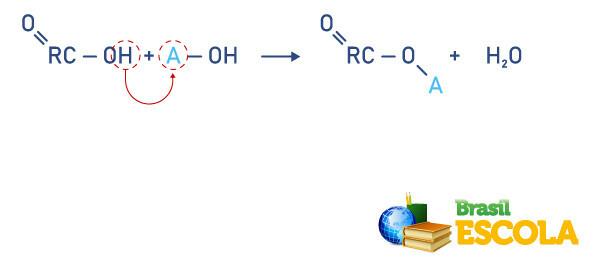
carboxylic acids react with bases, forming carboxylated salts or organic salts. See below the reaction with general formulas, consider R as an organic radical and A as a metal:
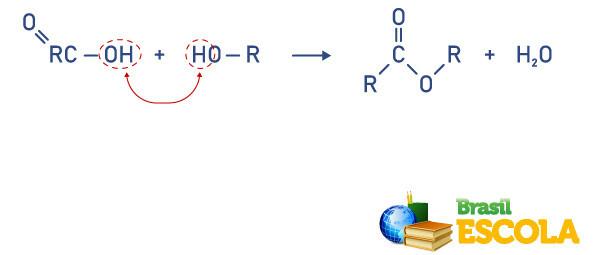
esterification reaction
This type of reaction is used mainly in the production of flavorings, it happens between a molecule of carboxylic acid and a alcohol, forming a ester and a molecule of Water.
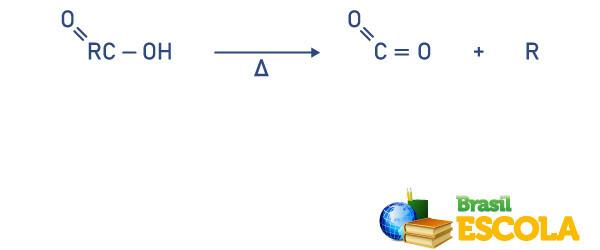
Decarboxylation reaction
In this type of reaction, the carboxyl removal from acid, having as a product the carbon dioxide it is a alkane or alkene, alkyne, organic molecule derived from the carboxyl-binding radical of the acid, see:
solved exercises
Question 1 - For carboxylic acids, tick the INCORRECT alternative.
A) Carboxylic acids are organic molecules that have a carboxyl functional group (-COOH).
B) The size of the carbon chain of a carboxylic acid defines characteristics such as appearance, melting point and density.
C) Carboxylic acids, when heated, can undergo a decarboxylation reaction, releasing carbon dioxide and a molecule from the organic group of the original molecule.
D) Propanic acid is also known as formic acid, this substance is present in the venom of red ants.
E) Tricarboxylic acids are organic compounds in which the molecule has three carboxylic groups. A tricarboxylic acid that is very present in our daily lives is citric acid, found in fruits such as lemons and oranges.
Resolution
Alternative D. Propanic acid is not the same as formic acid. The Iupac nomenclature for formic acid is: methanoic acid, a molecule consisting of only one carbon.
Question 2 - The following molecule is a monocarboxylic acid, sign the alternative which contains the proper nomenclature according to Iupac.

A) Propanic acid
B) Monobutanoic acid
C) Butanoic acid
D) 1-Methyl-butanoic acid
E) 3-Methyl-butanoic acid.
Resolution
Alternative E. The carbon count must start with the carboxyl carbon, so we will have a chain with four carbons corresponding to the prefix “but-”. The molecule is saturated, that is, it does not have double or triple bonds, so we will use the infix “-an-”. The methyl branch is located at carbon three, so the corresponding nomenclature will be 3-Methyl-Butanoic Acid.
By Laysa Bernardes Marques de Araujo
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/acidos-carboxilicos.htm

