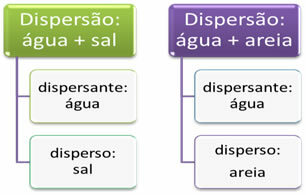
Dispersions refer to mixtures in general. For example, when we mix salt with water or salt and sand we get two dispersions. A substance that is spread, homogeneously (like salt in water) or heterogeneously (like sand in water), is called “dispersed”. Water, on the other hand, acts as a dispersant in these cases.
The main difference between these two dispersions is in the particle size of the disperse. While the sand grains can be seen with the naked eye, the salt particles are invisible. Based on this, dispersions can be classified into three main types, which are:
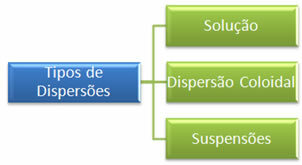
Note, in the table below, the difference in the size of the dispersed particles:

* Solutions: they are homogeneous mixtures in which we cannot see the dispersed particles even under a microscope. Some examples are the aforementioned salt mixed with water and also sugar mixed with water.
In the case of solutions, the disperse is called a solute and the dispersant is called a solvent. Solutions cannot be separated by any filtration process.
* Colloidal or Colloid Dispersions: some examples are mayonnaise and gelatin. Its particles are not visualized with the naked eye, so they are often confused with homogeneous systems, but they are actually heterogeneous, as can be seen with the use of microscopes. Its particles do not settle with the action of gravity, but only with ultracentrifuges.
Do not stop now... There's more after the advertising ;)
There are several types of colloidal dispersions, which are: sol, solid sol, gel, emulsion, foam and aerosol.
Separating this type of dispersion is not possible with filtration, but with a semi-permeable membrane. Its particles reflect and scatter light.
*Suspensions: they are heterogeneous systems, in which, even with the naked eye, it is possible to visualize their particles. Some examples are: sand in water, clay in water, milk of magnesia and calamine.
In the case of scatterings, light is also reflected. And its separation can be done by common filtration.

* Although some authors and researchers have proposed that colloidal particles have a size between 1 and 100 nm and that suspensions are greater than 100 nm, experimental evidence tends to increase this value to 1000 nm, and this value is the most accepted by most authors. However, its macroscopic behavior will really determine whether the mixture is colloidal or a suspension.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Types of Dispersions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/tipos-dispersoes.htm. Accessed on June 28, 2021.