As stated in the text "Chemical Equation”, to represent chemical reactions and understand the way they are processed, their quantitative and qualitative aspects, it was agreed to use chemical equations. Chemical equations contain symbols and numbers that show which substances are present and the appropriate proportions in which they react.
Through the interpretation of these equations, it is possible to find important data that help us to perform stoichiometric calculations. However, the first step says that the equation must be balanced, that is, it must have the total number of atoms of the reactants equal to that of the products. You can understand how to perform this balancing through the text "Equation balancing”.
Before, however, understand some important concepts in this case, such as the meaning of the terms: index, stoichiometric coefficient and mol. These terms will be explained below and give us a better glimpse of the quantitative part of the reaction:
- Index: is the number that indicates the number of atoms of each element present in chemical formulas. This number comes to the right of the element in question and appears subscript, that is, in a smaller font size.
Example:
H2O (chemical formula of water substance)
What does it mean:
- The elements that make up this substance are H (hydrogen) and O (oxygen).
- The index of each element indicates how many atoms of each are present in the water molecule formula:
H2O→ O-index: there is only 1 oxygen atom.
↓
H index: indicates that there are 2 hydrogen atoms.
Note that in the case of oxygen there is no written number, which means that there is only 1 atom of that element, as explained above.
Now, there are some cases where parentheses appear in formulas. How should we interpret this? See the example below:
Here3(DUST4)2(chemical formula of calcium phosphate substance)
- The Ca content indicates that there are 3 atoms of the element calcium present in the chemical formula;
- The P (phosphorus) and O (oxygen) are in parentheses, so the index that is outside, which in this case is 2, belongs to both. So, to find out how many atoms of each there are in the formula, you have to multiply their indices separately by the outer index. Note how this is done:

Do not stop now... There's more after the advertising ;)
P → index 1 O → index 4
P = 1. 2 O = 4. 2
P = 2 O = 8
So there are 2 phosphorus and 8 oxygen atoms.
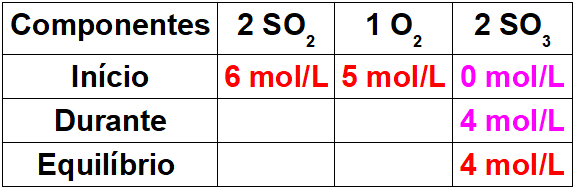
- Stoichiometric coefficient: is the number that comes before the formula to indicate the amount of each substance and the proportion of molecules participating in the reaction. Thus, as in the case of the index, when the coefficient is equal to 1, it is not necessary to write it down, as it is implied.
Example:
2H2 + 1O2 → 2 H2O (this water formation reaction is shown in the introductory figure)
↓ ↓ ↓
Reaction coefficients
In this equation, through the coefficients, it is shown that two molecules of hydrogen gas react with one of oxygen gas, to form two molecules of water as a product. Thus, the stoichiometric ratio of this reaction is 2:1:2.
To find out the total number of atoms of each element that are present in the reaction, it is necessary to multiply the coefficients by the indices of each element:
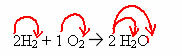
Reagents:
H = 2. 2 = 4 hydrogen atoms
O = 1. 2 = 2 oxygen atoms
Product:
H = 2. 2 = 4 hydrogen atoms
O = 1. 2 = 2 oxygen atoms
Note that you gave the same amount to the reactants and the products, which means the reaction is balanced correctly.
- Mol (amount of matter):in a chemical equation, the coefficients are considered to indicate the amount of mole or the amount of matter. So, in the previous case we have 2 moles of H2 reacting with 1 mol of O2, generating as products 2 moles of H2O.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Quantitative terms in a chemical equation"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/termos-quantitativos-uma-equacao-quimica.htm. Accessed on July 27, 2021.